Org. Synth. 2023, 100, 361-381
DOI: 10.15227/orgsyn.100.0361
Preparation of Pyrylium tetrafluoroborate (Pyry-BF4)
Submitted by Alejandro Gómez-Palomino, Clément Ghiazza, Julia Busch, Lucas Wagner and Josep Cornella*
1Checked by Thomas Leischner, Benjamin Thomas Jones, and Nuno Maulide
1. Procedure (Note 1)
Caution: chlorosulfonic acid and tetrafluoroboric acid diethyl ether complex are strong acids that react with water.
A. Potassium (1E,3E)-5-oxopenta-1,3-dien-1-olate (1) (Note 2). A 250 mL single-necked round-bottomed flask equipped with a cylindrical 7.5 cm Teflon-coated magnetic stirring bar is charged with a solution of potassium hydroxide (50.22 g, 895 mmol, 5.7 equiv) (Note 3) in deionized water (90 mL). The solution is sonicated until complete solubilization of KOH has been achieved (Figure 1A). The solution is vigorously stirred (under air with the flask loosely fitted with a rubber septum) and cooled in a dry ice-acetone bath (bath temperature = -20 ℃). Sulfur trioxide pyridine complex (25.00 g, 157 mmol, 1.0 equiv) (Notes 4 and 5) is added in five portions over the course of 20 min with vigorous stirring maintained (Figure 1B). The resulting thick yellow slurry (Figure 1C) is allowed to react at different temperatures as follows: 1) -20 ℃ (dry ice-acetone bath) for 1 h (yellow to orange slurry, Figure 1D); 2) allowed to warm to room temperature (22 ℃) for 4 h (slowly turns to black slurry, Figure 1E and 1F) (Note 6); 3) 40 ℃ (oil or water bath) for 0.5 h (dark solution); 4) cooled directly to 0 ℃ (ice bath) for 0.5 h. The solid product is collected by vacuum filtration using an oven-dried 125 mL glass fritted filter of porosity 4, washed with ice-cold acetone (2 × 50 mL) and MTBE (2 × 50 mL) (Note 7). The solid is transferred to a round-bottomed flask and further dried under high vacuum (4 x 10-3 mmHg) to give a dark brown solid (Figure 1G).
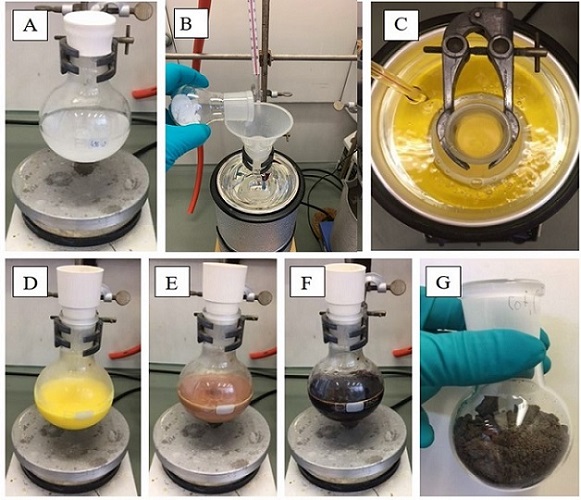
Figure 1. A) Solution of KOH in water; B) Addition of sulfur trioxide pyridine complex at -20 ℃; C) Reaction after addition of sulfur trioxide pyridine complex; D) Reaction after 1 h at -20 ℃; E) Reaction after 2 h at 22 ℃; F) Reaction after 4 h at 22 ℃; G) Crude material (photos provided by submitters)
A 2 L two-necked round-bottomed flask equipped with a cylindrical 5 cm Teflon-coated magnetic stirring bar and a reflux condenser is charged with the dark solid, 1 L of methanol (Note 8) and activated charcoal (2 g) (Note 9)(Figure 2A). The suspension is brought to reflux (oil bath, bath temperature = 70 ℃) during 15 min while open to air. The remaining black insoluble materials are removed by filtration of the hot mixture into a 2 L receiving round-bottom flask using a 125 mL glass fritted filter of porosity 4 under suction. The filter cake was washed with additional methanol (2 × 20 mL) giving a clear filtrate (Figure 1B). The clear filtrate (approx. 1.3 L) is concentrated at 40 ℃ by rotatory evaporation (60 mmHg) to a volume of approximately 30 mL, leading to the formation of a yellow/orange precipitate. The precipitate is collected by filtration using an oven-dried 125 mL glass fritted filter of porosity 4 under suction. The filter cake was immediately washed with ice-cold acetone (2 × 50 mL), followed by MTBE (2 × 50 mL). The solid was then transferred to a 100 mL round-bottom flask using a spatula, and dried under high vacuum (4 x 10-3 mmHg) to yield compound 1 as a yellow/orange solid (13.14 g, 96.5 mmol, 61%) (Note 10) (Figure 2C and 2D).

Figure 2. A) Purification setup; B) Removal of the activated charcoal; C) Filtration setup to collect product 1; D) Product 1 (photos provided by submitters)
B. Pyrylium tetrafluoroborate (2) (Note 11). A 250 mL two-necked round-bottomed flask, equipped with a cylindrical 5 cm Teflon-coated magnetic stirring bar, a glass stopper and an argon inlet, is carefully covered with aluminum foil (Note 12). The glass stopper is removed and potassium (1E,3E)-5-oxopenta-1,3-dien-1-olate (1) (3.00 g, 22 mmol, 1.0 equiv) is added to the flask using a plastic powder-addition funnel under positive argon flow, followed by the addition of anhydrous Et2O (50 mL) (Note 13). The flask is re-equipped with the glass stopper and the stopcock of the argon inlet is closed, sealing the system under an argon atmosphere. The suspension is vigorously stirred and cooled in a dry ice-ethanol bath (external temperature = -20 ℃) for at least 10 min (Figure 3A).
In parallel, a 50 mL single-necked round-bottomed flask equipped with a PE-rubber septum is charged with HBF4🞗OEt2 (20 mL, 147 mmol, 6.7 equiv) (Notes 14 and 15) with a syringe through the septum under positive argon flow and cooled to -20 ℃ in a dry ice-ethanol bath (Figure 3B). The septum is removed from the flask containing the cooled HBF4🞗OEt2 and the acid is added to the suspension of 1 (after reopening the argon inlet stopcock and. brief removal of the glass stopper) in one portion through the center neck with a glass funnel, turning the mixture to a dark brown color (see Note 15: rapid addition of HBF4🞗OEt2 to 1 is essential). After the addition is complete the glass stopper is added, and the argon inlet stopcock closed yet again. The cooling bath is removed, and the mixture is stirred for 16 h, during which time it was allowed to warm up slowly, ultimately reaching room temperature (22 ℃).
Figure 3. A) Reaction setup. B) Preparation of HBF4🞗OEt2 (photos provided by submitters)
After this time, the glass stopper is removed (with the argon inlet open, creating a positive flow of argon) and additional dry Et2O (100 mL) is added to the reaction flask using a syringe. At this point, the glass stopper is replaced by a septum, positive argon pressure is maintained and the mixture is stirred for 0.5 h at room temperature (22 ℃). Then, the mixture is cooled to -20 ℃ in a dry ice-ethanol bath over the course of 10 min. The stirring is stopped and a thick, dark purple slurry settles to the bottom of the flask, although it is difficult to observe the phase boundary between the slurry and the supernatant. The supernatant solvent is carefully removed by cannula with a PE-rubber tube and positive argon pressure (Note 16). After warming to 22 ℃, additional dry Et2O (50 mL) is added (using a syringe and needle pierced through the septum) to the remaining brown thick slurry and the mixture is vigorously stirred for 5 min. Then, the mixture is cooled to -20 ℃ in a dry ice-ethanol bath for 10 min, and the supernatant solvent is again removed by cannula with a PE-rubber tube and positive argon pressure. This rinsing process is repeated one more time, for a total of three rinses, after which the washed solids are allowed to warm to room temperature (22 ℃).
Acetonitrile (170 mL) (Note 17) is added to the mixture and the suspension is transferred by cannula to a 4 cm diameter glass fritted filter (Schlenk filter) of porosity 3 with a PE-rubber tube (inserted through a septum at the top of the filter) and positive argon pressure. After exchanging the septum with a glass stopper, the solution is filtered under argon pressure into a 1 L two-necked round-bottomed flask equipped with a cylindrical 5 cm Teflon-coated magnetic stirring bar and an argon line. The dark solid remaining in the filter is washed with CH3CN (2 × 10 mL, added after briefly removing the glass stopper), giving a clear dark red-brown filtrate solution (total volume: 190 mL) of crude pyrylium tetrafluoroborate. Black polymeric insoluble material remains in the filter (Note 18) (Figure 4).

Figure 4. Filtration set up; Solution of crude pyrylium tetrafluoroborate in the two-necked round-bottomed flask; Black polymeric insoluble material in the filter (photos provided by submitters)
The glass filter is then exchanged for a 1 L pressure-equalizing dropping funnel under positive argon flow, and purged with argon. Dry Et2O (550 mL) (Note 19) is charged into the pressure-equalizing dropping funnel, after briefly removing the glass stopper at the top, and added dropwise to the filtered solution (3-5 drops per second, gently stirring of the mixture) to form a fine precipitate (Figure 5). The mixture is then allowed to stir gently for an additional 10 min, before being transferred by cannula to a 4 cm diameter glass fritted filter (Schlenk filter) of porosity 3 under argon pressure, mounted on another 1 L two-necked round-bottomed flask equipped with an argon line. The heterogeneous mixture is filtered, and the retained solids are washed with Et2O (2 × 20 mL). The receiving flask is replaced with a round-bottomed flask (100 mL) and the solids are dried under high vacuum until a free-flowing powder is obtained. At this point, the solid material is transferred to an oven-dried vial (by pouring onto weighing paper and transferring to the vial), in which the material is subsequently further dried under high vacuum (4 x 10-3 mmHg) to yield compound 2 as an off-white to clear-brown solid (3.35 g, 19.9 mmol, 91%) (Notes 20 and 21) (Figure 5).
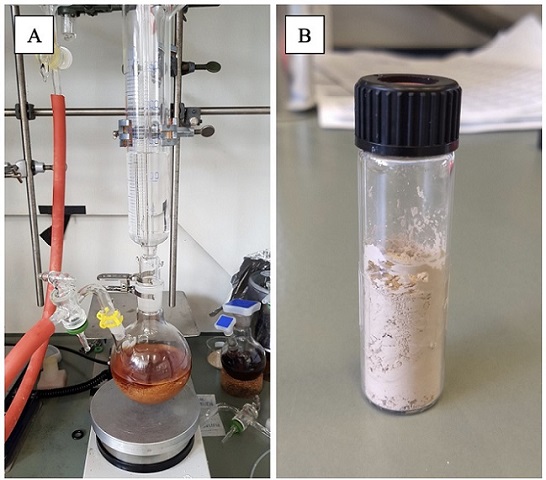
Figure 5. A) Glassware setup in which pure pyrylium tetrafluoroborate starts to precipitate; B) Product 2 after recrystallization (photos provided by submitters)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
pyridine,
chlorosulfonic acid,
dichloromethane,
acetone,
methyl tert-butyl ether,
potassium hydroxide,
sulfur trioxide pyridine complex,
ethanol,
methanol,
diethyl ether,
tetrafluoroboric acid diethyl ether complex and
acetonitrile.
2.
Potassium (1E,3E)-5-oxopenta-1,3-dien-1-olate (
1) was prepared according to a modified procedure by Becher reported in
Organic Syntheses.
2 High purity of the enolate is required to obtain good yields of
pyrylium tetrafluoroborate (
2).
3. The submitters purchased
potassium hydroxide from VWR Chemicals and used the material as received. The checkers purchased
potassium hydroxide from Fisher Chemicals and used the material as received.
4.
Sulfur trioxide pyridine complex (97%) was purchased from Sigma-Aldrich and used as received. The submitters observed lower yields if technical grade complex was used. However, reproducibility issues were often noticed when homemade
sulfur trioxide pyridine complex was prepared.
5. The checkers employed
sulfur trioxide pyridine complex (97%) from Sigma-Aldrich and obtained similar yields to that reported by the submitters, who prepared
sulfur trioxide pyridine complex according to a modified procedure from Sisler and Audrieth reported in
Inorganic Syntheses,3 which was performed as described herein. A 250 mL oven-dried three-necked round-bottomed flask (with at least one big neck) was equipped with a cylindrical 5 cm Teflon-coated magnetic stirring bar, thermometer, and a 25 mL pressure-equalizing dropping funnel. A vacuum adapter was attached to the funnel and the flask was evacuated under high vacuum (4 x 10
-3 mmHg) and refilled with argon (three cycles). Freshly distilled
pyridine (41.5 mL, 515 mmol, 2.0 equiv) and
CH2Cl2 (130 mL) were charged into the round-bottomed flask with a syringe. The solution is vigorously stirred and cooled in an ice-water-salt bath (internal temperature = 0 ℃).
chlorosulfonic acid (17.1 mL, 257 mmol, 1.0 equiv) was charged into the pressure-equalizing dropping funnel with a glass measuring cylinder and added dropwise to the
pyridine solution in a rate so that the internal temperature is kept below 5 ℃. The mixture was stirred for 30 min after the complete addition of the acid. The white precipitate was collected by filtration in an oven dried 125 mL glass fritted filter of porosity 4 under suction, quickly washed with ice-cold water (2 × 50 mL), ice-cold
acetone (2 × 25 mL) and
MTBE (2 × 25 mL), and dried under high vacuum (4 x 10
-3 mmHg) to give the title compound as a powdery white solid (33.40 g, 210 mmol, 82%). Purity of the product was determined to be 99 wt% by qNMR using
1,3,5-trimethylbenzene (99%, Alfa Aesar) as an internal standard. Noteworthy, despite identical spectroscopic data, reproducibility issues were encountered from one batch to another. We have observed that the synthesis of
1 is reproducible when the starting
pyridine🞗SO3 complex is a white powder. In contrast, deliquescent solids lead to poor yields (<20%). We attribute this to traces of polymeric material form the ring-opening process, which interfere in the second step, thus diminishing the final yield. Therefore, the submitters recommend using the commercially available
sulfur trioxide pyridine complex.
6. Aggregates might form during the reaction, and they must be broken with a spatula to ensure the stirring.
7.
Acetone, HPLC grade (99.5%) was purchased from Thermo Fisher, and
MTBE, reagent grade (>99.0%) was purchased from Sigma-Aldrich. Both were used as received.
8. The submitters purchased
methanol (99.8% GC) from Sigma-Aldrich and used the solvent as received. The checkers purchased
methanol (99.8% GC) from Fisher Chemicals and used it as received.
9. Activated charcoal was supplied by VWR-International GmbH and used as received.
pdf pdf pdf10. A second run on half scale provided 6.91 g (50.7 mmol, 65%) of product. Analytical data for
potassium (1E,3E)-5-oxopenta-1,3-dien-1-olate (
1) as obtained by the checkers:
1H NMR
pdf (600 MHz, DMSO-
d6) δ 8.67 (d,
J = 9.2 Hz, 2H), 7.04 (t,
J = 13.1 Hz, 1H), 5.12 (dd,
J = 13.0, 9.2 Hz, 2H);
13C NMR
pdf (151 MHz, DMSO-
d6) δ 184.4, 159.8, 106.2; IR (ATR): 2788, 1627, 1575, 1387, 1265, 1177, 1022 cm
-1; mp: >350 ℃; HRMS (ESI-): calcd. for C
5H
5O
2 [M-K
+]
-: 97.029505; found: 97.0296. Determined to be 87 wt% (checkers' full-scale run) or 86 wt% (checkers' half-scale run) by qNMR
pdf using
1,3,5-trimethoxybenzene (99%, Alfa Aesar) as an internal standard. In both cases, the purity was sufficient for good yields and excellent purities in the formation of
pyrylium tetrafluoroborate (
2).
11. All the glass material used in all the steps of the preparation was flame dried with a heat gun under high vacuum (4 x 10
-3 mmHg).
12. The reaction was performed in the dark to avoid polymerization.
13. The submitters purchased
Et2O from Emsure/Merck KGaA, which was distilled under argon from Mg/anthracene before use. The checkers purchased anhydrous
Et2O from Acros (99.5%) and used the material as received.
14. The submitters and checkers purchased
HBF4🞗OEt2 from Sigma-Aldrich and used the material as received. High quality of the acid is required to minimize the formation of polymers.
15. Protonation of
potassium (1E,3E)-5-oxopenta-1,3-dien-1-olate (
1) with 2 equiv of acid to form the oxonium ion is required for the construction of
pyrylium tetrafluoroborate (Figure 6). Partial protonation of
1 forms glutacon dialdehyde, which in turn leads to a purple solution. The submitters found that this color is an indicator of polymerization events as already suggested by Klages and Träger.
4 Therefore, it is important to go through the first step of the protonation process as fast as possible to avoid such polymerization. A large excess of
HBF4🞗OEt2 (6.7 equiv) at -20 ℃ in one portion moves the equilibrium as far as possible to the oxonium ion. Thus, the solution is not purple anymore (suggesting no polymerization) and is brown in color.
Figure 6. Formation of the pyrylium ion
16. As the checkers were not able to accurately distinguish between the liquid and solid phases, the tubing used for cannulation of the supernatant was fitted with additional filter paper, in order to prevent transfer of solid material. Figure 7 shows the tubing fitted with filter paper, which is secured in place using Teflon tape. In practice, the septum is pierced with the tubing, the filter paper is secured to the tubing, the septum is resecured, and cannulation is started.
Figure 7. Cannula equipped with filter paper (photo provided by checkers)
17. The submitters and checkers purchased
CH3CN from Sigma-Aldrich (99.8%) and stored the solvent over activated 3Å molecular sieves. The submitters did not observe improvement when distilled
CH3CN (CaH
2) was used.
18. Some amount of a black polymeric insoluble material remains in the filter. It turns white when exposed to air, but remains insoluble in
CH3CN and
acetone. The filter should be cleaned as soon as possible to avoid damage of the glass frit.
19. It is important to keep the ratio of
CH3CN:
Et2O to 1:2.5 ratio in order to obtain a high yield of
pyrylium tetrafluoroborate (
2). The overall volume of
CH3CN determines the amount of impurities in the isolated
pyrylium tetrafluoroborate (
2). More concentrated solutions afford
2 as a darker solid with reduced purity of 94%.
20. A second run on half scale provided 1.51 g (8.99 mmol, 82%) of product. The submitters report the following yields: from self-prepared
sulfur trioxide pyridine complex, 2.70 g, 16.1 mmol, 73%; from commercial
sulfur trioxide pyridine complex, 3.03 g, 18.1 mmol, 82%. Analytical data for
pyrylium tetrafluoroborate (
2) as obtained by the checkers:
1H NMR
pdf (600 MHz, CD
3CN) δ: 9.57 (dt,
J = 3.5, 1.8 Hz, 2H), 9.18 (tt,
J = 8.1, 1.8 Hz, 1H), 8.36 (ddd,
J = 8.1, 3.6, 1.5 Hz, 2H);
13C NMR
pdf (176 MHz, CD
3CN) δ: 169.4, 161.2, 127.7;
19F NMR
pdf (565 MHz, CD
3CN) δ: --151.76 (s, 1F), -151.82 (s, 3F);
11B NMR
pdf (193 MHz, CD
3CN) δ: -1.2; IR (ATR): 2781, 1611, 1524, 1386, 1260, 1192, 1027 cm
-1; mp: >300 ℃ (decomposition); HRMS (ESI+): calcd. for C
5H
5O
1 [M-BF
4]
+ 81.0335; found 81.0334; (ESI-): calcd. for [BF
4]
- 87.0035; found 87.0037. Determined to be 97 wt% (checkers' full-scale run) or >99 wt% (checkers' half-scale run) by qNMR
pdf using
1,3,5-trimethoxybenzene (99%, Alfa Aesar) as an internal standard.
21. The checkers observed considerable decomposition of
2 upon solubilization in deuterated-
acetonitrile that had not been pre-dried over molecular sieves.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Primary amino groups (R-NH
2) are ubiquitous functional groups present at the core of many biologically relevant molecules, such as DNA, vitamins, drugs, agrochemicals and fungicides. From a synthetic point of view, activation of the NH
2 group in a late-stage functionalization fashion would enable the use of this functionality as anchor points to build chemical complexity. To implement this strategy, chemoselective methods that target the NH
2 group without affecting other sensitive functionalities would be highly desirable. To this end, our group focused attention on the use of pyrylium salts as selective activators of amino groups.
5 In particular, we have developed a facile synthesis of Pyry-BF
4 (
2), which enable the chemoselective activation of aminoheterocycles and sulfonamides, which upon addition of different nucleophiles, permit direct modification of such scaffolds.
Capitalizing on the high reactivity of Pyry-BF
4 with NH
2 groups, we have developed a method that enables the activation of the NH
2 groups in aminoheterocycles, to forge the corresponding pyrdinium salts. These intermediates can now be engaged with a variety of S, O and N nucleophiles in a formal S
NAr reaction to forge the corresponding sulfones, thioethers, ethers or amines (Figure 8).
6 The protocol is characterized by a remarkably broad functional group compatibility, permitting the coupling in the presence of tertiary amines, piperidines, pyrrolidines, amides, halogens, trifluoromethyl groups, nitro groups, free alcohols and Michael acceptors, highlighting some advantages over predominant C-N activation strategies (diazonium and polyalkylation).
Figure 8. Functionalization of aminoheterocycles
Despite the synthetic challenges unlocked by the aforementioned strategy, the scope was mainly restricted to a handful of weak nucleophiles. Stronger nucleophiles, such as secondary or primary alkylamines, resulted in Zincke-type reactivity and transfer of the pyridine moiety. In addition, carbon centered nucleophiles irreversibly attacked the pyridinium scaffold. To tackle these hurdles, we developed a unified methodology that would broaden the scope of coupling partners. To this end, we developed a single flask preparation of heteroaryl chlorides from aminoheterocycles using cheap and commercially available chloride sources (Figure 9).
7 Although the reaction resembles the Sandmeyer reaction, the functional group tolerance is much broader. With the access to heteroaryl chlorides unlocked, the rich and robust chemistry of aryl chlorides can now be incorporated.
Figure 9. General functionalization of aminoheterocycles via the deaminative chlorination
In nature, deaminases are a class of enzymes responsible for the deaminative hydroxylation of nucleobases. Indeed, the interchange between an NH
2 and an OH group can reverse the H-bonding ability due an inverted tautomerism. Spurred by this bio-inspired process, we envisaged to match the enzymatic deaminative hydroxylation with
2. After an intensive screening, it has been found that the transient pyridinium could lead to the hydroxylated analogue via a mild Lossen-type rearrangement with a combination of commercially available hydroxamic acid and potassium carbonate (Figure 10).
8 Thus, complex bio-active substrates, including adenosine and cytidine, were interrogated and both successfully delivered the oxygenated products.
Figure 10. Enzyme-like deaminative hydroxylation
The synthetic utility of
2 is also exemplified in the late-stage modification of primary sulfonamides. Primary sulfonamides have largely been conceived to be an end-point in a synthetic route rather than a linchpin position.
9 Hence, we reported a method that repurposes primary sulfonamides, and converts them into useful electrophilic sites, primed for facile nucleophilic addition with various nucleophiles. The use of Pyry-BF
4 in combination with cheap and available MgCl
2 permits a mild substitution of the NH
2 group in primary sulfonamides to forge the corresponding sulfonyl chloride (Figure 11A).
10 To stress the utility of the protocol a preponderance of high-value heterocyclic frameworks bearing sensitive functionalities were interrogated. With the sulfonyl chloride formed
in situ, the addition of a nucleophile is within reach, following the traditional 1,2-addition.
Due to the growing interest in the synthesis of sulfonyl fluorides as activity-based probes for chemical biology,
11 we have also developed a one-pot strategy which gives access to the corresponding sulfonyl fluoride. Capitalizing on the
in situ formation of sulfonyl chlorides, addition of KF triggered an anion exchange, which led to the corresponding sulfonyl fluoride.
12 Due to the mild conditions of the protocol, this compound also enables the derivatization of complex sulfonamides as exemplified by the multitude of functional groups tolerated by the protocol. (Figure 11B).
Figure 11. Synthesis of sulfonyl chlorides (A) and sulfonyl fluorides (B) from primary sulfonamides
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Pyridine (110-86-1)
Chlorosulfonic acid (7790-94-5)
Potassium hydroxide; (1310-58-3)
Sulfur trioxide pyridine complex; (6412-87-3)
Potassium (1E,3E)-5-oxopenta-1,3-dien-1-olate; (1)(40418-44-8)
Activated charcoal; (7440-44-0)
Tetrafluoroboric acid diethyl ether complex; (67969-82-8)
Pyrylium tetrafluoroborate; (2) (80279-50-1)

|
Josep Cornella (Pep) obtained his Ph.D. in 2012 from Queen Mary University of London (UK), in the group of Prof. Igor Larrosa. He then pursued postdoctoral studies in the groups of Prof. Ruben Martin at the ICIQ (Spain) and Prof. Phil S. Baran at the Scripps Research Institute (USA). In 2017, he was selected by the Max-Planck-Society to create and lead the Laboratory for Sustainable Catalysis at the Max-Planck-Institut für Kohlenforschung (Germany), as a Max Planck Research Group Leader. His work focuses on the development of sustainable catalytic strategies to streamline organic synthesis. |

|
Alejandro Gómez-Palomino was born in Barcelona in 1990. He received his BSc in Chemistry from the University of Barcelona in 2012. That year, he joined the group of Prof. Fèlix Urpí and Prof. Pedro Romea at University of Barcelona, receiving his Master's Degree in 2013 and his Ph.D. degree in 2018. In 2019, he joined the group of Dr. Josep Cornella at the Max-Planck-Institut für Kohlenforschung (Germany). |

|
Clément Ghiazza obtained his Master's degree in organic chemistry at the University of Lyon (France) in 2016. He pursued a Ph.D. degree under the supervision of Dr. Anis Tlili and Dr. Thierry Billard at the same university until October 2019. Then, he held a postdoctoral fellowship from the Alexander von Humboldt foundation in the group of Dr. Josep Cornella at the Max Planck Institut für Kohlenforschung (Germany). In October 2022, he was appointed as a CNRS researcher at the Institut Lavoisier de Versailles (France). Clément was awarded the Dina Surdin prize by the French Chemical Society - Organic Chemistry Division (SCF - DCO) in 2020 for his Ph.D. |

|
Julia Busch, born in 1996 in Dorsten, completed her apprenticeship in synthetic chemistry in 2019 at the Max-Planck-Institut für Kohlenforschung (Germany). During this time, she worked in the group of Prof. Dr. Ferdi Schüth (heterogeneous catalysis) and in the group of Dr. Josep Cornella (sustainable catalysis), where she is still working as a laboratory technician, while earning her technician degree in evening school. |

|
Lucas Wagner, born in 1999 in Bochum, completed his apprenticeship in synthetic chemistry in 2021 at the Max-Planck-Institut für Kohlenforschung (Germany). During this time, he worked in the group of Prof. Benjamin List (homogenous catalysis) and in the group of Dr. Josep Cornella (sustainable catalysis), where he is still working as a laboratory technician in the fields of metal catalysis and organic synthesis. |

|
Thomas Leischner was born in 1989 in Kassel, Germany. He obtained his Master´s degree in chemistry from the University of Leipzig in 2015. Subsequently, he joined the group of Prof. Matthias Beller at the Leibniz Institute for Catalysis (LIKAT) as a PhD student. After successfully obtaining his Ph.D. degree in 2020, he joined the group of Prof. Nuno Maulide at the University of Vienna as a postdoctoral researcher. |

|
Benjamin Jones was born in 1993 in Newcastle Upon Tyne, UK. He obtained his Master´s degree in chemistry from Heriot-Watt University in 2017. He then joined the group Prof. John Bower at the University of Bristol as a Ph.D. student. After successfully obtaining his Ph.D. degree in 2022, he joined the group of Prof. Nuno Maulide at the University of Vienna as a postdoctoral researcher. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved