Org. Synth. 2023, 100, 394-403
DOI: 10.15227/orgsyn.100.0394
Preparation of (S)-3,3´-Bis(1-pyrrolidinylmethyl)-5,5´,6,6´,7,7´,8,8´-octahydro-1,1'-bi-2-naphthol
Submitted by Yu Hong, Yuan-Yuan Zhu, and Shuang-Xi Gu
*1Checked by Chibueze I. Onyeagusi and Kevin Campos
1. Procedure (Note 1)
(S)-3,3´-Bis(1-pyrrolidinylmethyl)-5,5´,6,6´,7,7´,8,8´-octahydro-1,1'-bi-2-naph-thol ((S)-A). A 250 mL three-necked round-bottomed flask is equipped with a stir bar (38.1 mm x 15.9 mm). One neck is fitted with an adapter for nitrogen flow and a second neck is fitted with a rubber septum. The flask is charged with (S)-H8BINOL (3.33 g, 11.3 mmol, 1.00 equiv) (Note 2) and paraformaldehyde (1.02 g, 33.9 mmol, 3.00 equiv) (Note 3). The center neck is equipped with a reflux condenser capped with a septum, and the flask is then evacuated and subsequently refilled with nitrogen. This process is conducted three times. A needle under nitrogen flow is inserted at the top of the condenser and the nitrogen line on one of the necks of the flask is quickly replaced with a rubber septum. Anhydrous ethanol (50 mL) (Note 4) is added by syringe through a septum, and the formation of a white suspension is observed. Pyrrolidine (2.8 mL, 33.9 mmol, 3.00 equiv) (Note 5) is then added to the reaction flask by syringe over one min, and the reaction mixture color changes to light brown during the course of the addition (Note 6) (Figure 1).
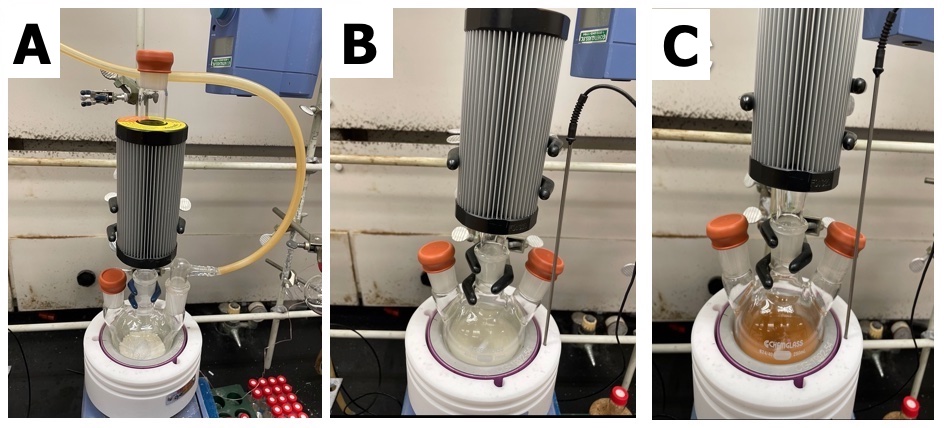
Figure 1. Photographs of reaction set up; A) Paraformaldehyde and (S)-H8BINOL being evacuated and backfilled; B) Reaction mixture after addition of ethanol; C) Reaction mixture after addition of pyrrolidine (photos provided by checkers)
A temperature probe is inserted into the flask and the reaction is heated to reflux in a heating block (95 ℃) for 13 h, and a clear orange solution is formed (Figure 2). Thin-layer chromatography (TLC) shows complete consumption of the starting material (S)-H8BINOL (Note 7) (Figure 2).
The flask is allowed to cool to room temperature, and the reaction mixture is poured into ice-cold water (250 mL) in a 1000 mL Erlenmeyer flask in one portion (Note 8), and white solids precipitate immediately. The precipitate is swirled in the flask to enhance dissolution of the precipitate and the resulting mixture is poured into a separatory funnel. The aqueous layer is extracted with dichloromethane (3 × 130 mL) in a 1000-mL separatory funnel. The combined organic layers are washed with brine (150 mL), dried over anhydrous Na2SO4 (20 g), filtered, and concentrated under reduced pressure (40 mmHg) on a rotary evaporator at 40 ℃ to afford a brown oil (Figure 3A). The brown oil is diluted with ethanol (25 mL) and then transferred to a 250 mL round-bottomed flask. The solution is stirred for 5 min, which facilitated the precipitation of the solid crude product (Figure 3B).
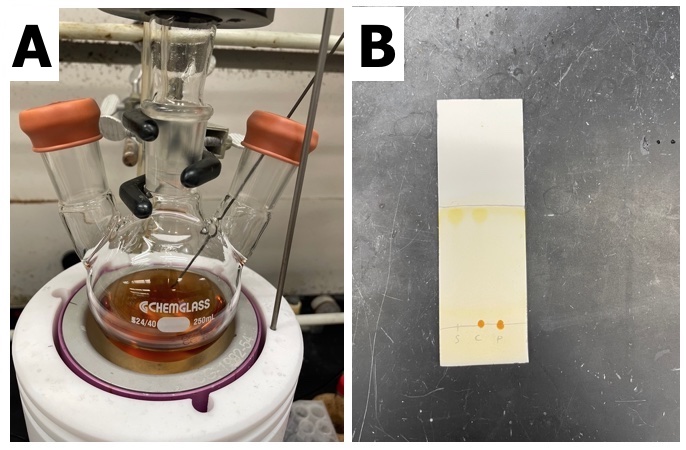
Figure 2. A) Reaction mixture after heating for 13 h; B) TLC Plates (100% ethyl acetate) for reaction mixture at complete conversion. Left lane = (S)-H8BINOL; middle lane = co-spot; right lane = reaction mixture. TLC plate visualized using iodine stain (photos provided by checkers)
Figure 3. A) Crude reaction mixture after workup and concentration under reduced pressure; B) Crude reaction mixture after addition of ethanol and stirring for 5 min; C) Crude reaction mixture after heating up in ethanol to dissolve solids (photos provided by checkers)
The ethanolic suspension is heated in an open flask to 70 ℃ for 10 min to dissolve the crude solids, and then the solution is allowed to cool slowly to room temperature over the course of 16 h to allow for crystallization to occur (Note 9). The reaction mixture containing the crystals is then filtered under vacuum to collect the crystals. The crystals are washed with cold ethanol (15 mL) (Figure 4). The white solids obtained from the filtration are dried to a constant mass under vacuum (1-2 mmHg) for 18 h to give (S)-1 as a white and fluffy solid (3.88 g, 75% yield, >99% ee) (Notes 10, 11, and 12).
Figure 4. A) Recrystallization setup after cooling to room temperature; B) Filtration and recovery of crystalline product after recrystallization; C) White solids after vacuum-drying overnight (photos provided by checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out regarding each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
(S)-H8BINOL,
paraformaldehyde,
pyrrolidine,
ethanol,
dichloromethane,
sodium sulfate,
ammonium hydroxide,
methanol, and
ethyl acetate.
2.
(S)-H8BINOL was prepared from
BINOL by partial hydrogenation with a high yield (>95%) under simple conditions.
2,3,4 Checkers used
(S)-H8BINOL (>99%, >99% ee) that was purchased from Ambeed Chemicals and used as received.
3.
Paraformaldehyde, powder (95%), was used as purchased from Tianjin Fuchen Chemical Reagents. Checker used
paraformaldehyde, powder (95%) that was purchased from Sigma-Aldrich and used as received.
4. Anhydrous alcohol (>99.7%) was used as purchased from Sinopharm Chemical Reagents. Checker used
ethanol (>99.5%) that was purchased from Sigma-Aldrich and used as received.
5.
Pyrrolidine, liquid (99%), was used as purchased from Shanghai Macklin Biochemical Technology Co., Ltd. Checker used
pyrrolidine, liquid (>99.5%) that was purchased from Sigma-Aldrich and used as received.
6.
BINOL and its derivatives are susceptible to oxidation.
7. Thin Layer Chromatography (TLC) plates were purchased from the Qingdao Ocean Chemical Plant Branch. Checkers used pre-coated TLC-plates (Merck & Co., Inc. TLC silica gel 60 F254). The plates were pretreated with ammonia before use according to the following method: the TLC silica plates were immersed in the mixture of
ammonium hydroxide and
methanol (1:5 V/V) for 1 min for pre-treatment. Then the TLC silica plates were taken out and allowed to dry in air. TLC analysis was then conducted with 100%
ethyl acetate as the mobile phase, and the TLC plates were visualized with iodine. The R
f of the product is about 0.01, while R
f of H
8BINOL is 0.96 (Figure 2B).
8. Ice-cold water (250 mL) is made of 125 g crushed ice and 125 mL water.
9. A large amount of precipitation occurs quickly as the flask cools to room temperature.
10. Characterization data for
(S)-1:
1H NMR
pdf (500 MHz, CDCl
3) δ: 6.74 (s, 2H), 4.04 (d,
J = 13.7 Hz, 2H), 3.66 (d,
J = 13.7 Hz, 2H), 2.79-2.72 (m, 4H), 2.65-2.63 (m, 8H), 2.41 (dt,
J = 17.4, 6.1 Hz, 2H), 2.23 (dt,
J = 17.4, 6.1 Hz, 2H), 1.81-1.65 (m, 16H);
13C NMR
pdf (126 MHz, CDCl
3) δ: 152.7, 135.4, 127.5, 126.9, 124.0, 59.0, 53.5, 29.3, 27.0, 23.7, 23.4, 23.3. [α]
D25 -43.2 (
c 1.0, DMF); IR (film): 2919, 2873, 2855, 2830, 2806, 2653, 1610, 1581, 1450, 1389, 1340, 1321, 1290, 1256, 1120, 1095, 875, 836, 720 cm
-1; mp 164-166℃ (lit.
1 159-160 ℃). HRMS (ESI) calculated for C
30H
41N
2O
2 ([M+H]
+):
m/z 461.3168, found: 461.3165. The purity of
(S)-1 was determined to be 99.0% by quantitative NMR
pdf analysis, using
1,3,5-trimethoxybenzene as an internal standard.
11. A second reaction performed by the checkers provided 3.65 g (70%) of the product with >99%
ee.
12. An Agilent 1290 chromatographic instrument was used for HPLC analysis. A Phenomenex Lux-Cellulose-4 column (part. No. 00F-4490-E0, particle size: 3 μM, column size: 4.6 mm × 150 mm) was eluted with gradient of a mixed solvent system of 10 mM
Na2B4O7 (at pH 9.2) and
acetonitrile at 1.0 mL/min. The retention times of the
R- and
S-enantiomers were determined to be as follows: t
(R)-1 = 25.7 min. and t
(S)-1 = 24.2 min.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
1,1´-Bi-2-naphthol (BINOL) and its derivatives have been extensively used for asymmetric catalysis
5,6 and fluorescent recognition of chiral molecules.
7 Derivatives of the partially hydrogenated BINOL, H
8BINOL, have also been studied and shown improved enantioselectivity over BINOL in many instances.
8,9 Thus, H
8BINOL derivatives are interesting ligands for asymmetric catalysis.
10 The title compound, (S)-1, was synthesized by a concise and facile one-pot synthetic method. In the new procedure, environment-friendly ethanol was used as an efficient solvent to replace the previously reported chloroform
2 and dioxane
10 solvents to prepare the H
8BINOL derivative.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Paraformaldehyde; (30525-89-4)
Pyrrolidine; (123-75-1)
Anhydrous alcohol; (64-17-5)
(S)-5,5´,6,6´,7,7´,8,8´-Octahydro-1,1´-bi-2-naphthol [(S)-H8BINOL]; [1,1´-Binaphthalene]-2,2´-diol, 5,5´,6,6´,7,7´,8,8´-octahydro-, (1S)-; (65355-00-2)
(1S)-5,5',6,6',7,7',8,8'-Octahydro-3,3'-bis(1-pyrrolidinylmethyl)[1,1'-binaphthalene]-2,2'-diol; [1,1´-Binaphthalene]-2,2'-diol, 5,5´,6,6´,7,7´,8,8´-octahydro-3,3´-bis(1-pyrrolidinylmethyl)-, (1S); (1224593-78-5)

|
Yu Hong obtained his master's degree in Wuhan Institute of Technology in June 2022 under the supervision of Dr. Yuan-Yuan Zhu and Dr. Shuang-Xi Gu, focusing on the development of novel chiral fluorescent probes and chiral catalysts. |

|
Yuan-Yuan Zhu got a B.S. degree from Wuhan Institute of Technology (WIT) in 2004 and a M.S. degree from Hubei Research Institute of Chemistry in 2007. She obtained her PhD at Shanghai Jiao Tong University in 2011. She worked at Wuhan Institute of Technology from 2012. From September 2017 to October 2018, she was a visiting scholar in the Department of Chemistry, University of Virginia. She was appointed as an associate professor in 2019. |

|
Shuang-Xi Gu received a B.S. degree from Wuhan Institute of Technology (WIT) in 2003 and a M.S. degree from Hubei Research Institute of Chemistry in 2006. He obtained a PhD degree in 2012 at Fudan university. Then, he worked at WIT in the same year. From 2014 to 2015, he worked as a postdoctoral fellow at University of Virginia (UVa). From 2017 to 2018, he conducted academic research at UVa again as a visiting scholar. He was appointed as a professor in WIT in 2021. |

|
Chibueze Onyeagusi is a Senior Scientist in the Process Chemistry group at Merck. Currently, he works with a team focusing on the development of commercial processes for small molecule API candidates. Chibueze is a native of Lagos, Nigeria. He obtained his B.S. in Chemistry from Southeastern Louisiana University in 2016 and earned his Ph.D. in Organic Chemistry from Duke University in Durham, NC under Prof. Steven Malcolmson in 2022 |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved