Org. Synth. 2023, 100, 404-417
DOI: 10.15227/orgsyn.100.0404
Preparation of (S)-5,5ʹ,6,6ʹ,7,7ʹ,8,8ʹ-Octahydro-1,1'-bi-2-naphthol [(S)-H8BINOL]
Submitted by Jiong Chen, Yuan-Yuan Zhu* and Shuang-Xi Gu*
1Checked by Jacob Waldman and Kevin Campos
1. Procedure (Note 1)
(S)-5,5ʹ,6,6ʹ,7,7ʹ,8,8ʹ-Octahydro-1,1'-bi-2-naphthol [(S)-H8BINOL] ((S)-1). (S)-1,1ʹ-Bi-2-naphthol ((S)-BINOL, 5.00 g, 17.46 mmol, 1.00 equiv) (Note 2), 10 wt% palladium on activated carbon (Pd/C) containing 50% H2O (0.93 g, 0.437 mmol, 0.025 equiv as Pd) (Note 3) and anhydrous ethanol (50 mL) (Note 4) are added at room temperature to a 100 mL autoclave (Note 5) equipped with an overhead stirrer with a pitched-blade impeller (Note 6), pressure gauge, internal thermowell for use with a thermocouple probe, gas inlet and outlet valves, and a pressure relief device (e.g., rupture disk). The vessel is pressurized with nitrogen (80 - 100 psig), vented, and refilled three times to purge air/oxygen before repeating three times with hydrogen (H2) to 80 - 100 psig to purge nitrogen (Note 7). From a regulated gas supply, the autoclave is refilled with hydrogen to 200 psig (Note 8) (Figure 1). The reaction mixture is then heated to 70 ℃ for 1 h without stirring. Once the reaction temperature of 70 ℃ is reached, the pressure is raised to 250 psig, stirring (500 rpm) is started, and the reaction is allowed to proceed at this temperature for 18 h.

Figure 1. Experimental set up. Regulated hydrogen supply from source tank on right. Pressure relief device line as thicker, steel braided tubing to exhaust manifold, and insertion gas inlet and vent through thinner metal line (photo by checkers)
The reaction mixture is allowed to cool to 25℃, and the remaining hydrogen is slowly vented through an appropriate vent line directly into the exhaust system of the fume hood over ~5 min. The vessel is pressurized with nitrogen (80 - 100 psig) and vented three times to purge residual hydrogen. Reaction completion is verified by thin layer chromatography (TLC) showing the starting material, (S)-BINOL, is completely consumed (Note 9). The resulting mixture is filtered with the assistance of vacuum (30 mmHg) through a short pad of filter aid (e.g., Celite) that is wetted with ethanol (Notes 10 and 11), and the filter cake is washed with anhydrous ethanol (3 × 5 mL). The combined clear, colorless filtrate and washings are collected in a tared 100 mL round-bottomed flask (Figure 2A). The filtrates are concentrated to dryness under reduced pressure at 50 ℃ bath temperature, adjusting the vacuum as necessary to approximately 20 mmHg in order to avoid bumping. The crude product is obtained as a white solid (Figure 2B).
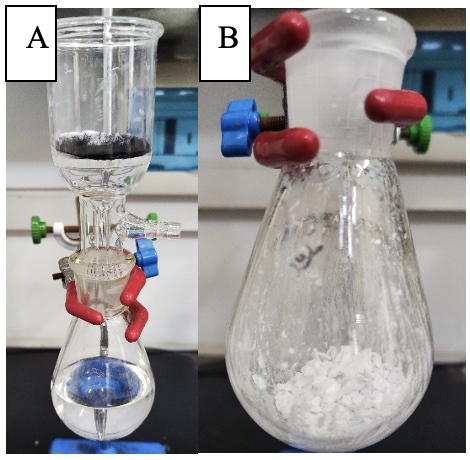
Figure 2. A) Combined filtrates from filtration and the washings; B) Crude product (S)-1 (photos provided by submitter)
A magnetic stir bar is added to this flask and the crude product is thoroughly scraped from the walls of the flask before hexanes (40 mL) (Note 12) are added to the flask. The flask is equipped with a condenser under an atmosphere of nitrogen, and the slurry is stirred (500 - 700 rpm) for 1 h at room temperature to perform trituration (Figure 3). The resulting slurry is vacuum filtered, and the filter cake is dried under vacuum (50 ℃, <100 mmHg) (Note 13) for 12 h or until a constant mass is achieved to afford the desired product (S)-1 (4.17 g, 81%) as a white solid (Notes 14 and 15) (Figure 3B), with >99% ee and >97 wt% purity (Notes 16, 17 and 18).
Figure 3. A) Trituration of crude product (S)-1 at room temperature; B) Purified product (S)-1 (photos provided by checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not be limited to) an evaluation of the potential hazards associated with
(S)-1,1ʹ-Bi-2-naphthol,
10% palladium on activated carbon,
ethanol,
hydrogen, and
n-hexane, as well as the proper procedures for handling
hydrogen gas under high pressure.
Hydrogen gas is extremely flammable, odorless, colorless, and is lighter than air and tends to rise and can form explosive mixtures in air in concentrations as low as 4% and as high as 74%, which is easily achievable in confined spaces such as fume hoods even with adequate flow velocity. A hydrogen detection system is highly recommended. Where in-built hydrogen detection systems are unavailable, personal, hand-held devices are commercially available (proper calibration and battery checks must be performed before each use). If a pressure vessel must be refilled during a reaction, precautions must be taken to prevent over-pressure, injury, or damage. A regulated gas supply must be used and all piping from the gas supply must be rated for the pressure to be applied. Before opening the gas supply, the regulator should be turned down and the pressure slowly raised to the desired set point. Once this is complete, the valve into the reaction vessel can be slowly opened and gas admitted. Once the vessel's pressure has been increased appropriately, the valve to the vessel should be shut and the gas supply tank valve shut off. Awareness of surplus gas in the line between the regulator and the vessel should be made and this gas may be left in the line until the experiment is complete and no further gas is necessary before safely venting this gas into an appropriate exhaust system.
Pressure vessels of any material of construction present several risks that must be understood by the user to prevent accident or injury as part of the initial risk assessment. Before use, be sure that the pressure rating of the vessel exceeds the pressure intended for use. Inspection prior to each use for damage should be carried out and the sealing surfaces must be thoroughly inspected and cleaned. If possible, a pressure test with an inert gas (e.g., nitrogen, helium) should be performed to ensure gas-tightness at the working pressure expected during the reaction. As an added precaution, a rupture disk or pressure relief apparatus designed to open well below the absolute pressure rating of the vessel should be installed to prevent catastrophic rupture or explosion in the event of an over-pressurization event. The outlet from the over-pressure safety system should be vented away from the user and preferably directly into the exhaust system. Special care must be exercised when heating any closed system to ensure that the final pressure at operating temperature remains below the absolute pressure rating of the vessel and any pressure relief system in place. Ideally, a partial pressurization should be performed at room temperature before applying heat and the final pressure applied once the vessel has stabilized at reaction temperature.
Metal catalysts, such as palladium on carbon, can spontaneously catch fire when exposed to air and this characteristic is far more significant after the catalyst has been exposed to hydrogen as the metal will adsorb a significant quantity of the gas and may be wet with a flammable solvent. After filtration and rinsing of the catalyst with a suitable process solvent, the spent catalyst must never be allowed to dry out and should be saturated in water and stored thereunder to prevent accidental overheating and fire. Additionally, proper disposal of catalysts should be performed, generally by submerging in water, the catalyst, any filter aids, and funnels (disposable plastic funnels are preferred).
Catalyst materials are sold either dry or water wet. Water wet catalysts are preferred due to their lower chances of catching fire. Water content can vary widely between lots and manufacturers and are typically in the range of 40 - 65 wt% water wet. When determining mol ratio of active metal, this water must be taken into account.
2. The checkers used
(S)-BINOL, white powder (95%+) as purchased from Matrix. Submitters used
(S)-BINOL, white powder (99%), as purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.
3. The checkers used 10 wt% palladium on carbon, 50 wt% water wet, which was used as purchased from Johnson-Matthey. The submitters used 10%
Pd/C, black powder containing 57.58%
H2O, which was used as purchased from Wuhan GeAo Chemical Technology Co., Ltd.
4. The checkers used anhydrous
ethanol from Sigma-Aldrich (>99%) as received. The submitters used anhydrous
ethanol (>99.7%) as received from Sinopharm Chemical Reagents.
5. The checkers used a 100 mL vessel. The submitters used a 200 mL autoclave. No difference in reactivity or productivity was observed during checking using the 100 mL vessel.
6. If overhead stirring is not possible, magnetic stirring was found to work equally well but led to grinding of the catalyst, which would serve to increase the surface area and reduce particle size. This could increase the likelihood of a fire once filtered, as well as increase the likelihood for the catalyst to break through the filter.
7. As described above, the checker exchanged gases by pressurizing the vessel and venting. The submitters exchanged gases by evacuation of the gases by vacuum and refilling to atmospheric pressure. No difference in purge efficiency was observed. Due to the inability to observe solvent evaporation or boiling in opaque, non-glass pressure vessels, it cannot be known if the solvent has bumped or otherwise vigorously boiled, possibly into the supply or vent lines when using the vacuum method; therefore, the checkers recommend use of the pressurization and venting procedure.
8. The submitters' procedure called for an initial pressure of 754 psig (5.2 Mpa, 52.0 bar) prior to heating. Due to the temperature increase, the internal pressure rose to approximately 5.7 MPa (827 psi, 57.0 bar). The autoclave was to be refilled to 5.2 MPa (754 psi, 52.0 bar)
H2 when the pressure dropped to 4.0 Mpa (580 psi, 40.0 bar) during the reaction. This refilling process was conducted one time. With the lower pressure described above, a regulated gas supply from a bottle or external reservoir was needed to maintain pressure throughout the reaction.
9. The checkers used precoated glass TLC plates (2.5 cm x 7.5 cm x 250 μm silica gel thickness with F254 indicator) purchased from EMD Chemicals. Reaction progress was monitored by TLC, eluting with 3:1
hexane:
ethyl acetate, visualizing with 254 nm ultraviolet light and/or iodine vapor. The R
f for
(S)-BINOL was 0.23 and for
(S)-H8BINOL was 0.31. Two impurities had R
f values of 0.35 and 0.50, respectively (Figure 4).
Figure 4. TLC plates after elution in 3:1 hexanes : EtOAc, visualized with iodine (left) and 254 nm UV illumination (right) (photos provided by checkers)
10. The filter aid should be added to the funnel dry and excess
ethanol added and the filter aid stirred to create an even slurry. The solvent was allowed to pass through the filter under gravity or gentle vacuum just to the top surface of the filter aid, at which time the side of the filter funnel should be gently struck with a piece of rubber tubing or other soft component to help completely settle the filter aid. Once the filter aid is compressed into a packed cake, a piece of filter paper should be placed on top to prevent the reaction mixture from eroding the cake and leading to potential catalyst breakthrough. The use of funnels with bare frits and no filter aid or the use of filter paper alone are not recommended.
11. All catalyst waste should be kept submerged in water prior to proper disposal.
12. The checkers used
hexanes used as purchased from Sigma-Aldrich. The submitters used
n-hexane as purchased from Sinopharm Chemical Reagents.
13. If a vacuum oven or similar equipment is not available, the product may be dried at room temperature after breaking up the cake into a powder by pulling vacuum through the product on the filter with a tent of
nitrogen on top. A plastic bag held to the funnel with a rubber band with a
nitrogen hose placed between the filter funnel and the plastic bag (Figure 5).
Nitrogen flow should be adjusted so that it exceeds vacuum flow so that the tent does not get pulled into the product bed.
Figure 5. Filtration with a nitrogen tent (photos provided by checkers)
14. The above procedure was equally effective in converting both
(R)-BINOL and (±)-BINOL to the corresponding (
R)- and (±)-H
8BINOL products, respectively. Characterization data for the product
(S)-5,5ʹ,6,6ʹ,7,7ʹ,8,8ʹ-Octahydro-1,1'-bi-2-naphthol [
(S)-H8BINOL,
(S)-1]: mp 160 - 162 ℃ (lit.
2, 3 160 - 161 ℃)
1H NMR
pdf (500 MHz, CDCl
3) δ: 7.06 (d,
J = 8.4 Hz, 4H), 6.82 (d,
J = 8.3 Hz, 4H), 4.60 (br. s, 2H), 2.75 (t,
J = 6.3 Hz, 4H), 2.23 (ddt,
J = 66.0, 17.4, 6.3 Hz, 4H), 1.71 (dm,
J = 29.8 Hz, 8H).
13C NMR
pdf (125 MHz, CDCl
3) δ: 151.5, 137.3, 131.1, 130.2, 119.0, 113.1, 29.3, 27.2., 23.1, 23.1. HRMS (ESI): Calculated for C
20H
23O
2+ ([M+H]
+):
m/z 295.1698, found: 295.1694. IR (film): 3472, 3375, 2927, 1586, 1471, 1426, 1286, 1247, 1194, 1151, 827 cm
-1.
15. A second reaction on larger scale provided 8.92 g (84% yield) of the product.
16. The checkers assessed enantiopurity using HPLC analysis. Enantiomeric purity was determined using a Chiralpak IC column (150 mm x 4.6 mm x 3 μm), eluting with an isocratic mixture of 9:1
hexanes:
isopropanol (by volume) at 1.0 mL/min at 25 ℃. The retention times of the product H
8BIBNOL
R- and
S-enantiomers were t
(R) = 3.41 min and t
(S) = 7.06 min, respectively. The retention times for the starting material BINOL R- and S-enantiomers were t
(R) = 4.41 min and t
(S) = 5.80 min, respectively.
17. Quantitative NMR
pdf purity was determined by completely dissolving 107.9 mg
(S)-H8-BINIOL with 25.1 mg of
1,3,5-trimethoxybenzene (99.9%) internal standard in CDCl
3 using a 10 sec FID delay to permit complete relaxation of all protons. Final wt% was determined by taking the sum of all protons in the product except the hydroxy signals (4.7 ppm) and the aromatic protons in the internal standard (6.1 ppm).
18. In the event that purity is low, a second trituration as described above can be carried out to further purify the product.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
1,1'-Binaphthyl-based chiral molecules have been extensively employed in asymmetric catalysis
4, 5, 6, 7 and molecular recognition.
7, 8, 9, 10 In the past two decades, a number of groups have used the enantiomerically pure 1,1'-bi-2-naphthol (BINOL) as the starting material to prepare BINOL-based fluorescent probes for chiral recognition.
7 5,5',6,6',7,7',8,8'-octahydro-[1,1'-binaphthalene]-2,2'-diol (H
8BINOL), the selectively partially hydrogenated BINOL, and other derivatives based on this structural motifs have also been investigated and shown higher enantioselectivity than BINOL-based probes in many cases.
11,12 Therefore, it is expected that the investigation of H
8BINOL-based probes might yield probes with significant fluorescent properties. Herein, a concise and efficient synthetic method for the preparation of (
S)-H
8BINOL from enantiomerically pure (
S)-BINOL is described. In this method, the work-up procedure is simple, and the product is provided in high chemical and enantio-purity.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
(S)-1,1ʹ-Bi-2-naphthol [(S)-BINOL]; S-(-)-2,2'-Dihydroxy-1,1'-binaphthyl [(S)-BINOL]; (18531-99-2)
10% palladium on activated carbon [Pd/C]; (7440-05-3)
Anhydrous ethanol; (64-17-5)
(S)-5,5ʹ,6,6ʹ,7,7ʹ,8,8ʹ-Octahydro-1,1ʹ-bi-2-naphthol [(S)-H8BINOL]; [1,1ʹ-Binaphthalene]-2,2ʹ-diol, 5,5ʹ,6,6ʹ,7,7ʹ,8,8ʹ-octahydro-, (1S)-; [(S)-H8BINOL]; (65355-00-2)

|
Jiong Chen obtained his master's degree in pharmaceutical engineering in Wuhan Institute of Technology (WIT) in 2021 and then worked in Zhejiang Huahai Pharmaceutical Co., Ltd. from 2021 to 2022. In Sept. 2022, he returned to WIT to study for his doctorate under the supervision of Prof. Shuang-Xi Gu. |

|
Yuan-Yuan Zhu earned a B.S. degree from Wuhan Institute of Technology (WIT) in 2004 and a M.S. degree from Hubei Research Institute of Chemistry in 2007. She obtained his Ph.D. at Shanghai Jiao Tong University in 2011. She worked at Wuhan Institute of Technology from 2012. From September 2017 to October 2018, she was a visiting scholar in the Department of Chemistry, University of Virginia. She was appointed as an associate professor in 2019. |

|
Shuang-Xi Gu received a B.S. degree from Wuhan Institute of Technology (WIT) in 2003 and a M.S. degree from Hubei Research Institute of Chemistry in 2006. He obtained a Ph.D. degree in 2012 at Fudan university. Then, he worked at WIT in the same year. From 2014 to 2015, he worked as a postdoctoral fellow at University of Virginia (UVa). From 2017 to 2018, he conducted academic research at UVa again as a visiting scholar. He was appointed as a professor in WIT in 2021. |

|
Jacob Waldman is a Scientist in the department of Chemical Engineering Research and Development (CERD) in the Hazardous Reaction Lab (HRL) at Merck. His career focus is planning and executing hazardous reactions that would be unsafe or dangerous in a standard laboratory environment due to toxicity, pressure, or corrosivity. He works with scientists throughout Merck to provide the highest quality and safest methods for performing these reactions on scales from milligrams to kilograms at all stages of project development. Jacob obtained his B.S. from the University of Rochester, under the advisement of Dr. Robert K. Boeckman, Jr., and his M.S. from the Pennsylvania State University, with Dr. Steven M. Weinreb, obtaining degrees in synthetic organic chemistry at both institutions. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved