Org. Synth. 2024, 101, 1-20
DOI: 10.15227/orgsyn.101.0001
Synthesis of Secondary Benzylic Alcohols by Reductive Arylation of Aldehydes: α-Phenyl-6-quinolinemethanol
Submitted by Gilian T. Thomas, Eric S. Isbrandt, and Stephen G. Newman*
1Checked by Christopher C. Nawrat and Kevin Campos
1. Procedure (Note 1)
A. 1,5-Bis(p-benzotrifluoride)-3,7-bis(cyclohexyl)-1,3,5,7-diazadiphosphacyclo-octane, PC2yNArCF32 (1) A 200 mL Chemglass heavy-walled round-bottomed pressure vessel (CG-1880-R) containing a 2.5 cm x 1 cm egg-shaped stir bar are oven-dried overnight (120 ℃) and transported into the glove box (Note 2). The flask is charged with paraformaldehyde (4.61 g, 152 mmol, 2.3 equiv) (Note 3). A plastic syringe is used to add cyclohexylphosphine (8.8 mL, 66 mmol, 1.0 equiv) (Note 4 and 5), and a plastic syringe is used to dilute the slurry with 40 mL of reagent alcohol (Note 6). The vessel is then sealed with a Teflon cap, removed from the glove box, and placed in a pre-heated 85 ℃ mineral oil bath overnight (400 rpm) (Note 7). The initially heterogeneous mixture is gradually transformed into a clear homogeneous solution (Figure 1).
Figure 1. Reaction mixture after heating at 85 ℃ overnight (photo provided by checkers)
After cooling the flask to 20 ℃, the flask is moved back into the glove box. A plastic syringe is used to add 4-aminobenzotrifluoride (8.3 mL, 66 mmol, 1.0 equiv) (Note 8) over approximately 10 sec to the stirring reaction mixture. The vessel is again removed from the glove box and heated at 85 ℃ (Figure 2A) for 6 h, during which time PCy2NArCF32 (1) gradually precipitates out of solution as a white solid (Figure 2B). The vessel is transferred into the glove box and 40 mL of anhydrous acetonitrile (Note 9) is added into the flask to induce further crystallization. The pressure flask is then sealed with its associated Teflon cap and transferred to the glove box freezer for storage at -20 ℃ for 48 h (Note 10).
Figure 2. A) Reaction mixture immediately after addition of 4-aminobenzotrifluoride. B) Reaction mixture after heating at 85 ℃ for 6 h (photos provided by the authors)
The vessel is then removed from the freezer and uncapped inside the glove box. Its contents are poured into a 60 mL fritted funnel, and the solvent is removed by suction filtration (Note 11). The solid is washed with 35 mL of hexanes (Note 12) followed by 200 mL of anhydrous acetonitrile, which was added in 20 mL portions. The solid is agitated with a metal spatula during rinses to ensure thorough contact with the solvent. The wet solid is transferred into a tared 250 mL two-necked, round-bottomed flask (24/40 joints), sealed with a rubber septum in one joint and a glass gas adaptor with stopcock, and shipped out of the glove box. Residual solvent is removed under high vacuum (25 ℃, 2 mmHg) until dry (up to 48 h). The final product is obtained (15.0 g, 75%) as a white solid that should be stored under nitrogen (Figure 3) (Notes 13, 14, and 15).

Figure 3. Isolated 1,5-bis(p-benzotrifluoride)-3,7-bis(cyclohexyl)-1,3,5,7-diazadiphosphcycloctane PCy2NArCF32 (1) after removal of solvent under vacuum (photo provided by checkers)
B. α-Phenyl-6-quinolinemethanol (2). A 300 mL round-bottomed flask (24/40 joint) containing a 2.5 cm x 0.8 cm rod-shaped stir bar is charged with 6-iodoquinoline (5.10 g, 20.0 mmol, 1 equiv) (Note 16) and PCy2NArCF32 (1) (1.42 g, 2.40 mmol, 0.12 equiv) (Note 7) and transported into a glove box. Inside the glove box NiBr2(diglyme) (0.35 g, 1.00 mmol, 0.05 equiv) (Note 18) is weighed into the flask. The flask is subsequently sealed with a rubber septum and transported outside the glove box (Figure 4A). The flask is connected to a Schlenk manifold with a needle adaptor open to nitrogen. 2,2,6,6-Tetramethylpiperidine (TMP) (5.65 g, 40.0 mmol, 2.0 equiv) (Notes 19 and 20) (Figure 4B), anhydrous toluene (100 mL) (Note 21 and 22), benzaldehyde (2.12 g, 20.0 mmol, 1.0 equiv) (Note 23) (Figure 4C), and 1-phenylethanol (7.33 g, 60.0 mmol, 3.0 equiv) (Note 24) (Figure 4D) are sequentially (Note 25) added to the flask in one portion from plastic syringes through the septum while the solution is stirred (500 rpm). The flask is then placed into an oil bath pre-heated to 75 ℃ and allowed to stir (500 rpm) for 16 h.
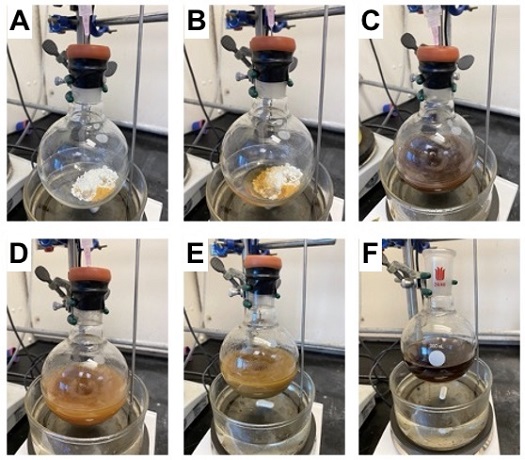
Figure 4. A) A 300 mL flask under N2 charged with solid reaction components. B) After addition of TMP. C) After addition of toluene and benzaldehyde. D) After addition of 1-phenylethanol. E) Reaction mixture after heating at 75 ℃ for 16 h. F) Completed reaction after solids have settled (photos provided by the authors)
After heating overnight, the heterogeneous reaction mixture is light brown/orange (Figure 4E). The flask is uncapped and allowed to cool to 20 ℃ (Figure 4F) before being concentrated by rotary evaporation (45 ℃, 75 mmHg) to a volume of approximately 10 mL. A Chemglass flash column (500 mL capacity, 24/40 joint, 25 cm L, 5.1 cm I.D.) is wet-packed with 170 g of silica (18 cm) (Note 26) in toluene, topped with 1.5 cm of sand, and the sample dissolved in toluene is added (Note 27). The column is eluted in a gradient using acetone (Note 28) and toluene. The gradient started with 500 mL of toluene, followed by 500 mL of 5% acetone in toluene collected into a flask. Collection of 20 mL column fractions begins when the column is eluted with 500 mL of 10% acetone in toluene, 500 mL of 15% acetone in toluene, 500 mL 20 % acetone in toluene, 500 mL 25% acetone in toluene, and finally 500 mL 30% acetone in toluene. Pure product is obtained in fractions 45-86, as determine by TLC analysis (Note 29). These fractions are combined and dried on the rotary evaporator (45 ℃, 38 mmHg), followed by high vacuum (25 ℃, 2 mmHg) to afford α-phenyl-6-quinolinemethanol (2) as a white powder (3.54 g, 75%) (Notes 30, 31, and 32). Traces of product along with impurities were identified in neighboring fractions (e.g. fractions 44 and 87) but were not collected.

Figure 5. Isolated α-phenyl-6-quinolinemethanol (2) (photo provided by the authors)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with paraformaldehyde,
cyclohexylphosphine,
reagent alcohol,
4-aminobenzotrifluoride,
acetonitrile,
hexanes,
6-iodoquinoline,
NiBr2(diglyme),
2,2,6,6-tetramethylpiperidine,
toluene,
benzaldehyde,
1-phenylethanol,
acetone, and
1,3,5-trimethoxybenzene, as well as the proper procedures for handling pyrophoric materials, and performing reactions in a sealed pressure vessel.
Cyclohexylphosphine (and primary phosphines in general) should be carefully handled under inert atmosphere to prevent contact with air. Any equipment or materials that come into contact with the primary phosphine should be transported out of the glove box in a sealed vessel and subsequently quenched in a bleach bath.
2. A glove box is required for the synthesis of
1 due to the pyrophoric nature of primary phosphines. This synthetic procedure has also been found to be sensitive to water, therefore use of a glove box and anhydrous solvents is necessary. It should also be noted that primary phosphines and many of their byproducts have a particularly strong and unpleasant smell.
3. Paraformaldehyde (95%) was purchased from Sigma-Aldrich and used as received.
4.
Cyclohexylphosphine was generously donated to the authors by Solvay as a neat liquid. The checkers purchased ampules of neat
cyclohexylphosphine from Strem Chemicals (min. 97%) that were opened within a glove box, where the reagent was then stored. As
cyclohexylphosphine is relatively volatile (boiling point 147-149 ℃), the checkers disabled circulation through the glove box catalyst bed and analyzer during use and enabled nitrogen atmosphere purge at a fairly high flowrate. Purging was continued for at least two h after
phosphine handling ceased in order to minimize contamination of the box.
5. All equipment and glassware that comes into contact with
cyclohexylphosphine should be sealed in an air-tight container and quenched in a bleach bath. The checkers used a large, wide mouth glass jar to collect contaminated materials in the glove box. Once sealed, removed from the glove box and transferred to an empty fume hood, commercial 3% bleach was carefully introduced to quench unreacted
phosphine.
6.
Reagent alcohol (anhydrous, ≤0.005% water) was purchased from Sigma-Aldrich and used as received. The checkers also used anhydrous Aldrich
reagent alcohol (which is ethanol containing 5%
i-PrOH and 5%
MeOH as denaturants), but the checkers obtained a comparable yield during a single run using pure anhydrous
ethanol. The checkers did not degas the reaction solvent.
7. For all operations involving heating a sealed reaction vessel, the checkers used a blast shield as a precaution against over-pressurization. The oil bath temperature was regulated by a thermocouple inserted into the oil itself.
8.
4-Aminobenzotrifluoride (98%) was purchased from Combi-Blocks and used as received. The checkers purchased this material from Sigma-Aldrich (99%).
9.
Acetonitrile (ACS grade) was purchased from Fisher Scientific and dried using a PureSolv solvent purification system. The checkers used anhydrous
acetonitrile as purchased from Sigma-Aldrich (99.8% with <0.005% water) and did not degas the solvent.
10. If a glove box freezer is unavailable, the checkers were able to obtain a lower but still useful yield (10.37 g, 52%) of the product by simply stirring at room temperature for 1 h after
acetonitrile addition, and then filtering the resulting suspension. Better recovery was achieved when the reaction was resealed after
acetonitrile addition, removed from the glove box and cooled in a regular lab freezer at -18 ℃ for 48 h. The checkers were then able to isolate by filtration under nitrogen atmosphere in a fume hood using a 250 mL Chemglass pressure filtration system (CG-1405-03) (Figure 6). The slurry was poured quickly into the filter funnel, which was immediately capped with nitrogen line.
Figure 6. Isolation 1 under nitrogen in the fume hood as an alternative to glove box filtration (photo provided by the checkers)
The solid product was dried under nitrogen flow and then quickly transferred to a two-neck round bottom flask and dried as described in the main text. This gave material in comparable yield and purity to that obtained in the glove box, seemingly unharmed by its brief contacts with air during filtration and drying. These options may offer an alternative for researchers who do not have access to a glove box freezer.
The use of a lab freezer followed by transfer of the cold slurry into the glove box for filtration is not recommended as this has the potential to introduce significant amounts of moisture due to condensation on the cold glass.
11. For filtration operations in the glove box, the checkers used a vacuum line pass-through into the building vacuum system (~20 mmHg). If an equivalent vacuum source is not available, the checkers used a hand-operated siphon pump, which is recommended as an alternative. A large gas-tight syringe (>50 mL recommended) can also be used as a makeshift vacuum source, although repeated operation can become tedious with large wash volumes. As the solid is further dried under high vacuum, it is not essential to completely remove all traces of the wash solvents at this point..
12.
Hexanes (HPLC grade) were purchased from Fisher Scientific and used as received. The checkers used anhydrous
hexanes as purchased from Sigma-Aldrich (99.8% with <0.005% water) and did not degas the solvent.
13. The product exists as two stable, separable conformers (presumably resulting from the slow pyramidal inversion of trialkylphosphines) and while the exact ratio isolated appears to vary somewhat batch to batch, it was typically found to be between 2:1 and 3:2. The minor conformer appears to be the more soluble of the two, and on one occasion the checkers were able to isolate a second crop of pure minor conformer from the cold mother liquors from the first filtration. Some overlap is present between the two species in the proton NMR spectrum. Major conformer:
1H NMR
pdf (500 MHz, CDCl
3) δ: 7.40 (d,
J = 8.9 Hz, 4H), 6.66 (d,
J = 8.8 Hz, 4H), 4.35 (t,
J = 14.0 Hz, 4H, major), 3.58 (dd,
J = 15.1, 5.0 Hz, 4H, major), 1.91-1.61 (m, 12H), 1.41-1.26 (m, 10H).
13C{
1H} NMR
pdf (126 MHz, CDCl
3) δ: 148.0, 126.5 (q,
J = 3.7 Hz), 125.2 (q,
J = 270.1 Hz), 118.0 (q,
J = 32.6 Hz), 111.9 (t,
J = 3.2 Hz), 55.5 (d,
J = 20.7 Hz), 34.9 (d,
J = 13.1 Hz), 29.4 (d,
J = 10.5 Hz), 27.2 (d,
J = 8.8 Hz), 26.41.
19F{
1H} NMR
pdf (470 MHz, CDCl
3) δ: -60.9, -61.0.
31P{
1H} NMR
pdf (202 MHz, CDCl
3) δ: -39.3. Minor conformer:
1H NMR
pdf (500 MHz, CDCl
3) δ: 7.44 (d,
J = 8.7 Hz, 4H), 6.90 (d,
J = 8.7 Hz, 4H), 3.98 (ddd,
J = 14.4, 3.9, 1.9 Hz, 4H), 3.73 (t,
J = 14.4 Hz, 4H), 2.00-1.59 (m, 12H), 1.49-1.26 (m, 10H);
13C{
1H} NMR
pdf (126 MHz, CDCl
3) δ: 151.6, 126.4 (q,
J = 3.7 Hz), 125.1 (q,
J = 270.5 Hz), 119.0 (q,
J = 32.7 Hz), 112.9 (t,
J = 2.6 Hz), 49.3 (dd,
J = 17.5, 6.8 Hz), 34.3 (d,
J = 11.8 Hz), 30.0 (d,
J = 12.3 Hz), 26.9 (d,
J = 9.8 Hz), 26.37;
19F{
1H} NMR
pdf (470 MHz, CDCl
3) δ: -61.1;
31P{
1H} NMR
pdf (202 MHz, CDCl
3) δ: -27.9.
14. A second reaction performed by the checkers on identical scale provided 14.8 g (74%) of product
1. In general, the authors report greater success in achieving good yields of
1 when the reaction is performed on a relatively large scale (>25 mmol).
15. The purity of
1 was determined to be 95 wt% by qNMR
pdf using
1,3,5-trimethoxybenzene (Sigma-Aldrich Reagent Plus ≥99%) as the internal standard.
16.
6-Iodoquinoline (98%) was purchased from Combi-Blocks and used as received.
17. The authors found that while
PCy2NArCF32 (
1) was generally stable when stored in the desiccator (that is, under dry air), certain batches were observed to have impurities present by
19F NMR over long term storage that are believed to result from oxidative decomposition. Therefore, it is recommended that the ligand is stored under inert atmosphere in either a glove box or a nitrogen-filled dry box to prevent deterioration over time. If the ligand is stored for prolonged periods of time outside of an inert atmosphere, the authors recommend that it be checked for decomposition prior to use. This can easily be done using
19F NMR spectroscopy, qualitatively or with an appropriate internal standard. The checkers found that after storing the ligand under air in a sealed vial on the bench, the purity was unchanged after three weeks (assessed by quantitative
1H NMR (
Note 15).
18.
NiBr2(diglyme) was purchased from Sigma-Aldrich and stored in a glove box. When stored outside the glove box, a gradual color change was observed that correlated to reduced yields.
19. Reagents were measured by mass into tared plastic syringes.
Toluene was measured by volume.
20.
2,2,6,6-Tetramethylpiperidine (98%) was obtained from Combi-Blocks and used as received. The checkers purchased
2,2,6,6-Tetramethylpiperidine from Sigma-Aldrich (≥99%) and used it as received.
21. The authors used
toluene dried using a PureSolv solvent purification system. The checkers used anhydrous
toluene as purchased from Sigma-Aldrich (99.8% with <0.005% water) and did not degas the solvent.
22.
Toluene was added in five 20 mL portions using a plastic 20 mL syringe.
23.
Benzaldehyde (sold as "purified by redistillation", ≥99.5%) was obtained from Sigma-Aldrich and used as received.
24.
1-Phenylethanol (98%) was purchased from Sigma-Aldrich and used as received.
25. Lower yields were found when
toluene was added prior to
TMP due to increased hydrodehalogenation of
6-iodoquinoline.
26. The authors used SiliaFlash Irregular Silica Gels F60, 40 - 63 μm, 60 Å purchased from SiliCycle. The checkers obtained similar results using EMD 60 Å, 230-400 mesh silica gel.
27.
Toluene (certified ACS) was purchased from Fisher Scientific and used as received.
28.
Acetone was purchased from Fisher Scientific and used as received.
29. The product (
2) was found to have an R
f of 0.36 (30%
acetone in
toluene (v/v)) when analyzed by TLC on silica.
30.
1H NMR
pdf (500 MHz, CDCl
3) δ: 8.75-8.72 (m, 1H), 8.09 (d,
J = 7.8, 1H), 7.97 (d,
J = 8.7 Hz, 1H), 7.87 (s, 1H), 7.63 (dd,
J = 8.8, 2.0 Hz, 1H), 7.41-7.25 (m, 6H), 6.01 (s, 1H), 3.96 (
br s, 1H);
13C NMR
pdf (126 MHz, CDCl
3) δ: 150.2, 147.7, 143.8, 142.5, 136.5, 129.5, 128.76, 128.74, 128.2, 127.9, 126.9, 124.7, 121.4, 75.9. Accurate Mass (EI) H
13C
16NO theoretical
m/z: 235.0992, found: 235.0998, spectral accuracy: 99.3%; mp 62 ℃.
31. A second reaction by the checkers provided 3.39 g (72%) of compound
2 as a white powder.
32. The purity of
2 was determined to be >97 wt% by qNMR
pdf using
1,3,5-trimethoxybenzene (Combi-Blocks 98%) as the internal standard. Note that the chemical shift of the hydroxyl proton is quite concentration dependent; it was observed between 3 and 4 ppm in typical samples.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
This procedure provides access to secondary alcohols via reductive coupling of aryl iodides and aldehydes, using 1-phenylethanol as the terminal reductant. Other ways of accessing these compounds typically include use of stoichiometric organometallic reagents. For example, one of the most common ways of synthesizing secondary alcohols from aldehydes is via the Grignard reaction. However, the air/moisture sensitivity and poor functional group tolerance of these organomagnesium-mediated processes leaves much room for improvement.
2 In situ formation of organometallic nucleophiles, for example as seen in the Reformatsky and Barbier procedures, are appealing but maintain use of stoichiometric metal additives.
3 In terms of catalysis, the Ni-catalyzed Nozaki-Hiyama-Kishi (NHK) reaction provides reductive coupling between organohalides and aldehydes facilitated by stoichiometric chromium.
4 While the breadth of methods for the reductive arylation of aldehydes continues to grow,
5 new protocols that overcome the need for stoichiometric metals remain rare.
6In 2021, our group reported several novel secondary alcohol structures accessed by the presented procedure, including
2 (Figure 7).
7 This synthetic method provides a method of directly using organohalides as a coupling partner, removing the need to prepare an organometallic nucleophile and avoiding the use of stoichiometric metals. The chemistry is facilitated by a unique 1,5-diaza-3,7-diphosphacyclooctane ligand (
1), which is well established for catalytic hydrogen splitting and formation, but is largely unused in organic synthesis.
8 These ligands are commonly abbreviated as P
R2N
R'2, where R and R' refers to the substituents on P and N, respectively. The ligand used herein, P
Cy2N
ArCF32, is derived from cyclohexylphosphine and 4-aminobenzotrifluoride. However, the synthesis is modular, and other derivatives can be made using alternative starting materials.
7 The crystal structure of a Ni(0)/P
Cy2N
ArCF32 complex illustrates that it is a bidentate ligand with both phosphines coordinated and both nitrogens uncoordinated.
9 The largest challenge with the synthesis of these ligands is the pyrophoric nature of monophosphines, which are best handled in a glove box. However, P
Cy2N
ArCF32 can be readily made on multi-gram scale, and is relatively stable to air and moisture. We believe these ligands have significant untapped potential for organic synthesis, and they may be valuable for a broad range of catalytic transformations not yet discovered.
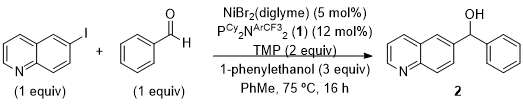
Figure 7. Reaction conditions used in the presented procedure to access α-phenyl-6-quinolinemethanol (2)
As shown in the selected scope in Figure 8, this nickel-catalyzed reductive coupling reaction shows broad functional group tolerance, enabling the coupling of both complex aryl iodides and aldehydes. The reaction works best when benzaldehyde derivatives are used as coupling partners. Aliphatic aldehydes could be used in many cases, such as in the synthesis of α-(phenylmethyl)-1-naphthalenemethanol (5), but the products are often contaminated with the corresponding ketone.
Figure 8. Selected scope of the nickel-catalyzed reductive coupling of aldehydes and aryl iodides
7To access these types of aliphatic-substituted alcohols, a redox-neutral variant of the reaction is recommended (Figure 9). Primary alcohols can be used instead of aldehyde substrates with the same catalyst, provided some minor changes are made to the reaction conditions. In particular, a higher catalyst loading is needed, the 1-phenylethanol reductant is removed, and the alcohol substrate is used in excess (up to 2.5 equiv).
Figure 9. Selected scope examples of the nickel-catalyzed redox-neutral coupling of aliphatic-substituted alcohols and aryl iodides
7
Appendix
Chemical Abstracts Nomenclature (Registry Number)
α-Phenyl-6-quinolinemethanol (2); (130343-63-4)
Paraformaldehyde; (30525-89-4)
Cyclohexylphosphine; (822-68-4)
Reagent Alcohol; (64-17-5)
4-Aminobenzotrifluoride; (455-14-1)
6-Iodoquinoline; (13327-31-6)
NiBr2(diglyme): Nickel(II) bromide 2-methoxyethyl ether complex; (312696-09-6)
2,2,6,6-Tetramethylpiperidine; (768-66-1)
Benzaldehyde; (100-52-7)
1-Phenylethanol; (98-85-1)

|
Gilian T. Thomas obtained her B.Sc. and M.Sc. at Carleton University. She then moved on to complete her Ph.D. at the University of Victoria under the supervision of Professor Scott McIndoe, studying reaction mechanisms using mass spectrometry. Her postdoctoral fellowship brought her to the University of Ottawa to work with Professor Stephen Newman, pursuing catalysis research and reaction discovery. |

|
Eric S. Isbrandt is from Ottawa, Canada and obtained his B.Sc. at the University of Ottawa. He is currently a Ph.D. candidate in the Newman group. Eric's research focuses on the application of 1,5-diaza-3,7-diphosphacyclooctanes as ligands to enable challenging transition metal catalyzed cross couplings. |

|
Stephen G. Newman grew up in Newfoundland, Canada and attended Dalhousie University, where he completed his B.Sc. He received a Ph.D. University of Toronto studying palladium catalysis in the laboratory of Mark Lautens and carried out postdoctoral studies in the lab of Prof. Klavs F. Jensen at the Massachusetts Institute of Technology where he investigated new continuous processes for chemical manufacturing. Stephen is currently an Associate Professor at the University of Ottawa where he holds the Tier 2 Canada Research Chair in Sustainable Catalysis. |

|
Chris Nawrat was born in Carshalton, England (near London) in 1986. He completed his Ph.D. studies at the University of Nottingham in 2012 working with Professor Chris Moody on several total syntheses. In 2013 he moved to the US to undertake postdoctoral studies in the group of Dave MacMillan at Princeton where he gained expertise in photo- and organocatalysis. Two years later, he joined Merck and Co., Inc. where he enjoys tackling challenging problems in drug development and is currently situated in the Discovery Process Chemistry group based in West Point, Pennsylvania. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved