Org. Synth. 2024, 101, 34-50
DOI: 10.15227/orgsyn.101.0034
Metal-Free Aziridination of Alkenes with Free Amines by Thianthrenation
Submitted by Ming-Shang Liu and Wei Shu*
1Checked by Michael D. Zott and Dirk Trauner
1. Procedure (Note 1)
A. Thianthrene S-oxide (1). Under an ambient atmosphere, a 500 mL round-bottomed flask equipped with a 3.0 cm x 0.75 cm rod-shaped teflon coated magnetic stirring bar (Figure 1a) is sequentially charged with thianthrene (21.63 g, 100.0 mmol, 1.00 equiv) (Note 2), DCM (200 mL, c = 0.5 M) (Note 3), sodium bromide (0.51 g, 5.00 mmol, 5.00 mol%) (Note 4), acetic acid (4.29 mL, 4.50 g, 75.0 mmol, 75.0 mol%) (Note 5), and iron(lll) nitrate nonahydrate (40.4 g, 100.0 mmol, 1.00 equiv) (Note 6) (Figure 1b). The reaction is left open to the atmosphere. The resulting reaction mixture is vigorously stirred (800 rpm) at 25 ℃ for 4 h. The reaction is diluted with water (200 mL) and is poured into a separatory funnel (Figure 2a). The aqueous and the organic layers are separated.
Figure 1. Reaction assembly for step A. (a) a 500 mL flask and a magnetic stirring bar; (b) reaction mixture after addition all of the materials (photos provided by authors)
The aqueous layer is extracted with DCM (2 x 50 mL). The organic layers are combined and washed with water (2 x 50 mL), then dried over Na2SO4 (40 g) (Figure 2b) and filtered through a glass funnel containing a plug of cotton wool. The sodium sulfate is washed once with DCM (50 mL). The filtrate is concentrated under reduced pressure (350 mmHg to 15 mmHg, 35 ℃) to a beige solid. The resulting solid is broken into smaller pieces with a spatula and stirred in EtOAc (50 mL) for 0.5 h (Note 7). The obtained microcrystals are collected by filtration on a 60 mL sintered glass funnel, washed with Et2O (2 x 30 mL) (Note 8), and dried in vacuo (0.75 mmHg) to afford 18.8 g (81%) of Thianthrene S-oxide (1) (Note 9) as white powder (Figure 2c).
Figure 2. Post-processing for step A. (a) extraction using dichloromethane; (b) the organic layer dried over Na2SO4; (c) final compound 1 (in a 200 mL flask) (photos provided by authors)
B. (E)-5-(4-Phenylbut-1-en-1-yl)-5H-thianthren-5-ium tetrafluoroborate (2). Under a nitrogen atmosphere, a 200 mL pear-shaped Schlenk flask equipped with a 3.0 cm x 0.75 cm rod-shaped magnetic stirring bar is charged with Thianthrene S-oxide (1) (9.74 g, 41.9 mmol, 1.05 equiv), 4-phenyl-1-butene (5.99 mL, 5.28 g, 40.0 mmol, 1.00 equiv) (Note 10), and MeCN (50 mL) (Note 11). After cooling to 0 ℃, trifluoroacetic anhydride (16.7 mL, 25.2 g, 120.0 mmol, 3.00 equiv, in 20.0 mL CH3CN) (Note 12) is added dropwise in 3 min (Figure 3a), followed by dropwise addition of TfOH (4.2 mL, 7.2 g, 48 mmol, 1.2 equiv, in 10.0 mL CH3CN) (Note 13) in 3 min (Figure 3b). The resulting lilac-colored mixture is stirred at 0 ℃ for 1.5 h followed by stirring at 25 ℃ for 1 h. The purple mixture is transferred to a 500 mL flask and concentrated under reduced pressure (150 mmHg to 20 mmHg, 40 ℃). The residue is diluted with CH2Cl2 (160 mL). A 3.0 cm x 0.75 cm rod-shaped stirring bar is added to the flask. The solution is cooled to 0 ℃, followed by careful addition of a saturated aqueous NaHCO3 solution (100 mL) in 5 min (Caution! Gas is evolved. See Note 14) (Figure 3c).
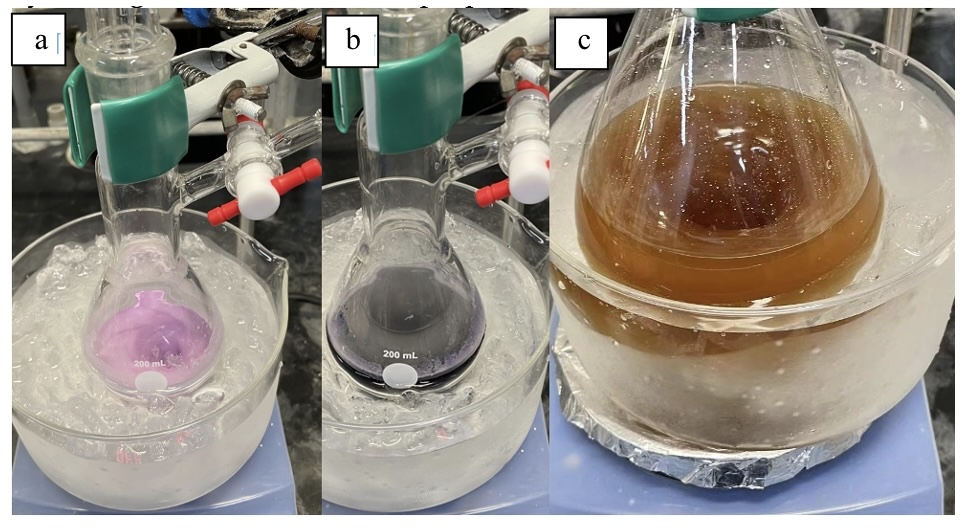
Figure 3. Reaction assembly for step B. (a) reaction mixture after addition of trifluoroacetic anhydride; (b) reaction mixture after addition of trifluoromethanesulfonic acid; (c) reaction mixture after quenched with saturated aqueous NaHCO3 solution (photos provided by authors)
The resulting mixture is vigorously stirred (500 rpm) at 25 ℃ for 5 min. Then, this mixture is poured into a 500 mL separatory funnel. The organic layer is separated and transferred to a 500 mL flask with a 3.0 cm x 0.75 cm rod-shaped stirring bar. Then, an aqueous solution of NaHCO3 and NaBF4 (10.0 g NaBF4 dissolved in 100 mL of saturated NaHCO3 solution) (Notes 15 and 16) is added to the flask. The resulting solution is vigorously stirred (500 rpm) for 10 minutes at 25 ℃. The mixture is poured into the 500 mL separatory funnel. The organic layer is separated and treated again with an aqueous solution of NaHCO3 and NaBF4 (10.0 g NaBF4 dissolved in 100 mL of saturated NaHCO3 solution). The mixture is poured into the 500 mL separatory funnel. The organic layer is separated and returned to the 500 mL flask. Then, the following procedure is performed for a total of four times: in the 500 mL flask with stirring bar, aqueous NaBF4 solution (50 mL H2O, 20.0 g NaBF4) is added to the organic layer to form a biphasic mixture that is vigorously stirred (500 rpm) for 20 min at 25 ℃. The mixture is poured into the 500 mL separatory funnel and the organic phase is separated. After four cycles, the organic layer is separated and dried over Na2SO4 (30.0 g) (Note 17), filtered, and the solvent is removed under reduced pressure (20 mbar, 35 ℃). The residue is purified by chromatography on silica gel (Note 18) (100 g) eluting with MeOH/CH2Cl2 (0-2%, v/v) (Note 19) (Figures 4a-c). All of the product-containing fractions (including mixed fractions) are collected and concentrated under reduced pressure (15 mmHg, 40 ℃) to afford the crude product as a viscous yellow oil. The oil is diluted with CH2Cl2 (5 mL) and transferred to a 250 mL Erlenmeyer flask equipped with a 3.0 cm x 0.75 cm rod-shaped stirring bar. While stirring (500 rpm), Et2O (80 mL) is added to precipitate the product. This mixture is left stirring for at least 2 h to ensure complete precipitation and to help pulverize the clumps of solid product into smaller microcrystalline material. This mixture is filtered over a medium porosity frit (60 mL), washed with additional Et2O (80 mL), and dried under vacuum (0.8 mmHg) to give (E)-5-(4-phenylbut-1-en-1-yl)-5H-thianthren-5-ium tetrafluoroborate (E/Z >10/1, 12.6 g, 73 %) (Note 20) as an off-white solid (Figure 4d).
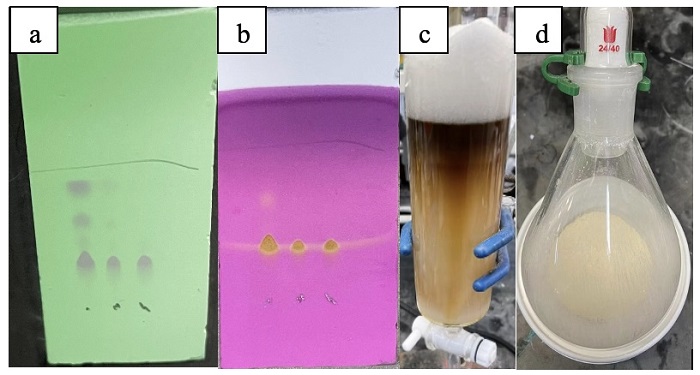
Figure 4. Post-processing for step B. (a) TLC of organic phase at end-of-reaction: reaction mixture (left) versus with final compound 2 (right) using 1:10 methanol: dichloromethane eluent, visualized under 254 nm UV lamp; (b) the same TLC as in (A) after staining with standard KMnO4 stain; (c) purification by column chromatography; (d) final compound 2 (in a 200 mL flask) (photos provided by authors)
C. 1-Octyl-2-phenethylaziridine (3). Under nitrogen atmosphere, a 200 mL pear-shaped Schlenk flask equipped with a 3.0 cm x 0.75 cm rod-shaped magnetic stirring bar (Figure 5a) is charged with (E)-5-(4-phenylbut-1-en-1-yl)-5H-thianthren-5-ium tetrafluoroborate (2) (5.85 g, 13.5 mmol, 1.00 equiv). The flask is equipped with a rubber septum and MeCN (110 mL) is added via syringe (Note 11). Next, Octylamine (2.68 mL, 2.09 g, 16.2 mmol, 1.2 equiv, in 20.0 mL CH3CN) (Note 21) is added to the solution via syringe in one portion (Figure 5b). The resulting mixture is stirred at room temperature for 5 min (Note 22). Under a counter flow of nitrogen, tBuONa (1.29 g, 13.5 mmol, 1.00 equiv) is added in one portion (Note 23) (Figure 5c) and the septum is replaced. The resulting solution is stirred at 25 ℃ for 12 h to give a purple mixture (Note 24), which is concentrated under reduced pressure (20 mmHg, 40 ℃).

Figure 5. Reaction assembly for step C. (a) a 200 mL flask and a magnetic stirring bar; (b) reaction mixture after addition of the Octylamine; (c) reaction mixture after addition of the sodium tert-butoxide (photos provided by authors)
The residue (Note 25) is purified by chromatography on silica gel (80 g) (Note 26) eluting with acetone/toluene (0-10%, v/v) (Figures 6a-c). The product-containing fractions are collected and concentrated under reduced pressure (20 mm Hg, 40 ℃) to give the 1-Octyl-2-phenethylaziridine (3) (2.31 g, 66%) (Note 27) as lightly yellow oil (Figure 6d).
Figure 6. Post-processing for step C. (a) TLC of organic phase at end-of-reaction: reaction mixture (left) versus with final compound 3 (right) using 1:9 acetone: petroleum eluent, visualized under 254 nm UV lamp; (b) the same TLC as in (a) after staining with standard KMnO4 stain; (c) purification by column chromatography; (d) final compound 3 (in a 10 mL vial) (photos provided by authors)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.htmL. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
thianthrene,
sodium bromide,
dichloromethane,
acetic acid,
iron(lll) nitrate nonahydrate,
ethyl acetate,
diethyl ether,
4-phenyl-1-butene,
acetonitrile,
trifluoroacetic anhydride,
trifluoromethanesulfonic acid,
sodium bicarbonate,
sodium tetrafluoroborate,
sodium sulfate anhydrous,
methanol,
octylamine,
sodium tert-butoxide,
petroleum ether,
acetone and silica gel.
2.
Thianthrene (98%) was purchased from Meryer and was used as received (authors). The checkers purchased
thianthrene (98%) from Ambeed and used it as received. Some
thianthrene remains undissolved after addition of the
dichloromethane.
3.
Dichloromethane (AR, >99.5%) was purchased from Adamas-beta (Shanghai Titan Scientific Co., Ltd.) and was used as received. The checkers purchased
dichloromethane from Fisher (>99.5%) and used it as received. The
dichloromethane was measured in a graduated cylinder (TD ± 1.0 mL) and carefully poured into the reaction flask.
4.
Sodium bromide (>99.0%) was purchased from Adamas-beta (Shanghai Titan Scientific Co., Ltd.) and was used as received. The checkers purchased
sodium bromide from TCI (>99.5%) and used it as received.
5.
Acetic acid (99.5%) was purchased from Xilong Scientific and was used as received. The checkers purchased
acetic acid(99.7%) from Spectrum Chemical and used it as received. The
acetic acid was measured using a disposable plastic syringe (expelled volume ± 5%) and carefully dispensed into the reaction flask.
6.
Iron(lll) nitrate nonahydrate (98.5%) was purchased from Mreda and was used as received. The checkers purchased iron(III) nitrate nonahydrate (>98%) from Fisher and used it as received.
7.
Ethyl acetate (AR, >99.5%) was purchased from Adamas-beta (Shanghai Titan Scientific Co., Ltd.) and was used as received. The checkers purchased
ethyl acetate (≥99.8%) from MilliporeSigma and used it as received.
8.
Diethyl ether (>99.5%) was purchased from Shanghai Lingfeng Chemical Reagent Co. Ltd. and was used as received. The checkers purchased
diethyl ether (≥99%) from Thermo Scientific and used it as received.
9.
Thianthrene S-oxide (
1) has the following physical and spectroscopic properties: mp 141-143 ℃;
1H NMR
pdf (600 MHz, CDCl
3) δ: 7.92 (d,
J = 7.8 Hz, 2H), 7.61 (d,
J = 7.6 Hz, 2H), 7.54 (t,
J = 7.6 Hz, 2H), 7.41 (t,
J = 7.6 Hz, 2H);
13C NMR
pdf (151 MHz, CDCl
3) δ: 141.6, 130.0, 129.2, 128.58, 128.57, 124.6; IR (film, cm
−1) 3049, 2915, 2847, 1434, 1248, 1117, 1075, 1023, 748, 451. The purity was determined to be 97.6% by quantitative NMR
pdf spectroscopy with
1,3,5-trimethoxybenzene as internal standard. HRMS (EI)
m/
z: Calcd. for C
12H
8OS
2 [M]
+: 232.0017, found: 232.0022. A second reaction by the checkers on an identical scale provided 19.47 g (84%) of the product.
10.
4-phenyl-1-butene (98%) was purchased from Macklin and was used as received. The checkers purchased
4-phenyl-1-butene (≥98.0%) from TCI and it was used as received.
11.
Acetonitrile was purchased from Xilong Scientific, and freshly distilled over calcium hydride (5 g CaH
2/500 mL
CH3CN‚ reflux 4 h) (authors). The checkers purchased anhydrous
acetonitrile (99.9%) over molecular sieves from Thermo Scientific and used it as received.
12.
Trifluoroacetic anhydride (99%) was purchased from Energy Chemical and was used as received. The checkers purchased
trifluoroacetic anhydride (≥99%) from Sigma Aldrich and used it as received.
13.
TfOH (99%) was purchased from Adamas-beta (Shanghai Titan Scientific Co., Ltd.) and was used as received. The checkers purchased
TfOH (≥98.0%) from TCI and it was used as received.
14. Initially, the solution should be added in a dropwise fashion due to the production and release of gas. After gas evolution has ceased, the remaining solution can be added in one-portion.
15. The addition of
NaBF4 to the mixture enhances the efficiency of anion exchange.
16.
NaBF4 (99%) was purchased from Bide Pharmatech and was used as received. The checkers purchased
NaBF4 (97%) from Thermo Scientific and it was used as received.
17.
Sodium sulfate (anhydrous) was purchased from Xilong Scientific and was used as received. The checkers purchased anhydrous
sodium sulfate from MilliporeSigma (>99.0%), and it was used as received.
18. Silica gel (Silica gel 90Å, 38-48 μm) was purchased from Nuo Tai Co., Inc. and used as received. The checkers purchased silica gel from SiliCycle 40 - 63 μm, 60 Å (R10030B) and it was used as received.
19. The column of silica (100 g) is wet packed in a 6 cm diameter x 30 cm height column using
dichloromethane (300 mL) (
Note 3). Then, the crude residue (solving in 20 mL
dichloromethane) is loaded onto the column. At that point, fraction collection (100 mL fractions, 27 x 200 mm) is begun, and elution is continued with 400 mL of
dichloromethane, then 600 mL of
methanol/
dichloromethane (1/100, v/v), then 1000 mL of
methanol/
dichloromethane (1/50, v/v). Fractions 7-21 were collected.
20.
(E)-5-(4-phenylbut-1-en-1-yl)-5H-thianthren-5-ium tetrafluoroborate (
2) has the following physical and spectroscopic properties: R
f = 0.3-0.4 (1:9
methanol-
dichloromethane; silica gel plate,
KMnO4); mp 117-122 ℃;
1H NMR
pdf (600 MHz, CDCl
3) δ: 8.21 (d,
J = 7.4 Hz, 2H), 7.83 - 7.79 (m, 2H), 7.78 - 7.71 (m, 2H), 7.67 - 7.61 (m, 2H), 7.24 (dt,
J = 14.6, 5.3 Hz, 1H), 7.16 - 7.06 (m, 3H), 7.02 (d,
J = 7.4 Hz, 2H), 6.41 (d,
J = 14.7 Hz, 1H), 2.75 (t,
J = 8.6 Hz, 2H), 2.64 - 2.49 (m, 2H);
13C NMR
pdf (151 MHz, CDCl
3) δ: 155.9, 139.5, 135.5, 134.5, 133.3, 130.3, 130.2, 128.6, 128.5, 126.4, 120.6, 110.3, 34.8, 33.3; IR (film, cm
−1) 3090, 3050, 3023, 1615, 1454, 1091, 1049, 1035, 752, 700, 503. The purity was determined to be 95.8% by quantitative NMR
pdf spectroscopy with
1,3,5-trimethoxybenzene as internal standard. HRMS (ESI)
m/
z: Calcd. for C
22H
19S
2 [M]
+: 347.0928, found: 347.0910. A second reaction by the checkers on an identical scale provided 11.57 g (67%) of the product.
21.
Octylamine (99.5%) was purchased from Bide Pharmatech and was used as received. The checkers purchased
Octylamine (≥98.0%) from TCI and it was used as received.
22. Premixing the
octylamine and thianthrenium salts is very important for this aziridination. The absence of premixing led to a low yield of this aziridination reaction. After 5 min, precipitation is occasionally observed.
23.
tBuONa (99%) was purchased from Adamas-beta (Shanghai Titan Scientific Co., Ltd.) and was used as received. The checkers purchased
tBuONa (97%) from Sigma Aldrich and it was used as received.
24. Occasionally, the mixture did not turn purple, but the yield was not affected.
25. The
thianthrene byproduct is sparingly soluble in
acetonitrile, so the majority of the
thianthrene can be removed prior to chromatography by triturating the concentrated reaction mixture with
acetonitrile and retaining the supernatant. However, this trituration was not used in this case.
26. The column of silica (80 g) is wet packed in a 6 cm diameter x 30 cm height column using
toluene (300 mL) (
Note 14). Then, the crude product (adsorbed on 8.0 g silica) is loaded onto the column. At that point, fraction collection (100 mL fractions, 27 x 200 mm) is begun, and elution is continued with 700 mL of
toluene, then 1000 mL of
acetone/ toluene (10/100, v/v). Fractions 8-18 were collected. The authors used a solvent system of 0-10%
acetone /
petroleum ether, but the checkers found that this resulted in coelution of
thianthrene.
Thianthrene is much more nonpolar (higher R
f) than the product, but it has low solubility in
petroleum ether, presumably resulting in the observed streaking.
27.
1-Octyl-2-phenethylaziridine (
3) has the following physical and spectroscopic properties: R
f = 0.2-0.3 (1:9
acetone-
hexanes; silica gel plate,
KMnO4);
1H NMR
pdf (600 MHz, CDCl
3) δ: 7.30 - 7.25 (m, 2H), 7.22 - 7.15 (m, 3H), 2.80 (ddd,
J = 13.8, 9.5, 6.0 Hz, 1H), 2.71 (ddd,
J = 13.8, 9.4, 6.5 Hz, 1H), 2.27 (ddd,
J = 11.5, 8.7, 6.4 Hz, 1H), 2.13 (ddd,
J = 11.5, 8.6, 5.8 Hz, 1H), 1.78 - 1.71 (m, 1H), 1.67 (ddt,
J = 13.6, 9.5, 6.5 Hz, 1H), 1.62 - 1.48 (m, 3H), 1.37 - 1.24 (m, 11H), 1.19 (d,
J = 6.3 Hz, 1H), 0.89 (td,
J = 7.1, 1.8 Hz, 3H);
13C NMR
pdf (151 MHz, CDCl
3) δ: 142.1, 128.6, 128.5, 125.9, 61.8, 39.8, 35.0, 34.0, 32.0, 30.1, 29.8, 29.4, 27.6, 22.8, 14.3; IR (film, cm
−1) 3028, 2924, 2854, 1707, 1496, 1454, 1078, 1031, 743, 697. HRMS (ESI)
m/
z: Calcd. for C
18H
29N [M]
+: 259.2300, found: 259.2291. The purity was determined to be 98.6% by quantitative NMR
pdf spectroscopy with
1,3,5-trimethoxybenzene as internal standard. A second reaction by the checkers on a similar scale provided 2.44 g (66%) of the product.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Aziridines are prevalent three-membered structural units in natural products and bioactive molecules.
2,3 The development of efficient methods to build aziridines represents one of the most challenging and contemporary subjects in organic synthesis.
4,5 Most classical and reliable methods to aziridines rely on the transition-metal-catalyzed aziridination of alkenes from nitrene precursors.
4,5,6 However, these methods require the use of suitable and activated nitrene precursors, such as azides and iminoiodinanes, in the presence of a transition-metal. These methods are typically limited to the use of explosive and hazardous nitrene precursors as well as additional steps for diazo synthesis and transition-metal residue removal. The synthesis of aziridines from free amines and alkenes offer a straightforward and appealing alternative strategy.
7 Recently, new methods utilizing electrochemical activation to facilitate oxidative cyclization have been developed by other groups.
8,9 In 2021, Noël developed an elegant example of electrochemical aziridination of internal styrenes with primary amines.
10 In 2021, Wickens reported electrochemical aziridination of terminal aliphatic alkenes with aliphatic primary amines in the presence of excess thianthrenium. In 2022, we reported a general and practical protocol for aziridination of alkenyl thianthrenes using primary amines, amides, sulfonamides, and carbamates as nitrogen sources.
11 The metal-free conditions allow for the direct aziridination of terminal and internal alkenes as well as aromatic and aliphatic alkenes.
12
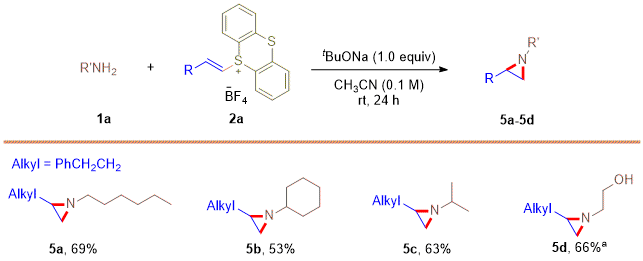
Figure 7. Substrate scope of amines. aK2CO3 (1.0 equiv) as base, DCM (0.1 M) as solvent
A representative scope of aziridination using primary amines is shown in Figure 7, where products 5a-5d were formed in good yields. In addition, primary carbonamides could be transformed into the desired aziridine products 6a-6d in good yields (Figure 8). The use of the primary amides and sulfinamides was also suitable for this aziridination reaction, yielding the products 6e-6g in 51%-78% yield.
Figure 8. Substrate scope of carbonamides and amides
The scope of aziridine formation when primary sulfonamides were used is shown in Figure 9. Electron-withdrawing and electron-donating substituted aryl sulfonamides were well-tolerated to give corresponding products in good yields (7a-7d, Figure 9). Heteroaromatic and aliphatic free sulfonamides worked well for this reaction, furnishing the corresponding aziridines in 71-77% yields (7e-7g, Figure 9). Notably, sulfamide derivatives can undergo mono- or bis-aziridination with alkenyl thianthrenium salt to deliver aziridination products 7h in 75% yield and 7i in 67% yield. In terms of the alkene scope, thianthrenation of gaseous alkenes could be carried out to provide access to aziridines 7j and 7k in 88% and 95% yields, respectively. Alkenyl thianthrenium salts with a pendant alkene, bromide or ester were all compatible in the reaction and delivered the desired aziridines in 65-85% yields (7l-7n, Figure 9). Moreover, thianthrenium salts derived from cyclic and acyclic internal aliphatic alkenes were amenable to this aziridination, affording the aziridines in 51-86% yields (7o-7r, Figure 9). A styrene-derived thianthrenium salt also worked well for this reaction, delivering the aziridine product in 54% yield (7s, Figure 9).
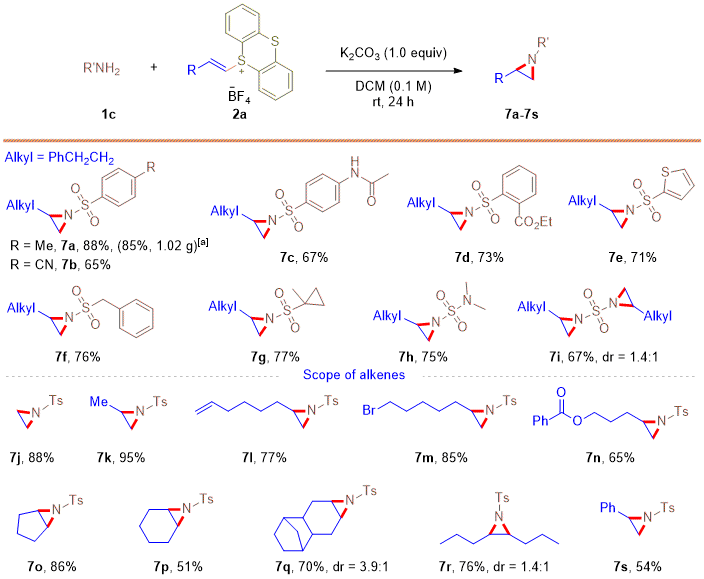
Figure 9. Substrate scope of sulfonamides and alkenes
In conclusion, a general and practical protocol for intermolecular aziridination of alkenes via the corresponding thianthrenes was achieved. The operationally simple conditions tolerate a broad spectra of alkenes and nitrogen sources to provide direct and general access to aziridines from readily available starting materials.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Thianthrene: Thianthrene; (92-85-3)
Iron(lll) nitrate nonahydrate: Iron nitrate nonahydrate; (7782-61-8)
4-Phenyl-1-butene: 4-Phenyl-1-butene; (768-56-9)
Trifluoroacetic anhydride: Trifluoroacetic anhydride; (407-25-0)
TfOH: Trifluoromethanesulfonic acid; (1493-13-6)
NaHCO3: Sodium bicarbonate; (144-55-8)
NaBF4: Sodium tetrafluoroborate; (13755-29-8)
Octylamine: Octylamine; (111-86-4)
tBuONa: Sodium tert-butoxide; (865-48-5)

|
Ming-Shang Liu was born and raised in Shandong province, China. In 2014, he received his B.S. in Chemistry from Shanxi University. He received his Ph.D. degree in the Shanghai Institute of Organic Chemistry (joint student with ShanghaiTech University) under the direction of Professor Yiyun Chen in 2019. Then, he joined the Shu group at the SUSTech where he is working on new organic coupling reactions. |

|
Dr. Wei Shu is a Principal Investigator in the department of chemistry at Southern University of Science and Technology. He received his bachelor's degrees at Nankai University and continued his graduate studies at Shanghai Institute of Organic Chemistry, where he obtained his Ph.D. degree under the guidance of Profs. Shengming Ma and Guochen Jia. He started his independent research at SUSTech in 2018 after several postdoctoral stints at Massachusetts Institute of Technology, Princeton University, and University of Zurich. His research interests focus on racial chemistry and asymmetric synthesis with an emphasis on first-row transition-metal catalysis and photochemistry. |

|
Michael D. Zott was born in Louisiana, USA and completed his undergraduate studies in 2018 (B.S. Chemistry, Physics Minor) at the Georgia Institute of Technology, where his research experiences ranged from theoretical chemistry (with Prof. C. David Sherrill) to experimental inorganic chemistry (with Prof. Henry La Pierre). His doctoral research was completed at the California Institute of Technology in 2023 under the supervision of Prof. Jonas C. Peters, where he developed electrocatalysts for small-molecule activation and organic chemistry applications. He is now a postdoctoral scholar with Prof. Dirk Trauner at the University of Pennsylvania, where he is investigating the total synthesis and photopharmacology of steroidal compounds, broadly defined. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved