Org. Synth. 2024, 101, 51-60
DOI: 10.15227/orgsyn.101.0051
Cobalt-catalyzed Radical Olefin Isomerization: Retrocycloisomerization of (-)-Caryophyllene oxide to (-)-Humulene oxide II
Submitted by Eleanor M. Landwehr and Ryan A. Shenvi*
1Checked by Emily P. Chen and Sarah E. Reisman
1. Procedure (Note 1)
A. (-)-Humulene oxide II (2). A 500 mL round-bottomed flask equipped with a 1-inch stir bar is fitted with a rubber septum, flame-dried under vacuum, and allowed to cool under a positive pressure of nitrogen. To the flame-dried flask equipped with stir bar is added benzene (Note 2) (136 mL, 0.1 M) and then (-)-caryophyllene oxide (1.0 equiv, 3.00 g, 13.6 mmol) (Note 3). To this stirring solution, Co(salentBu,tBu)Cl (1.0 mol%, 87 mg, 0.14 mmol) is added (Note 4). The dark green solution is degassed by bubbling argon via a metal needle (16 gauge) for 20 min at room temperature with stirring (400 rpm) (Note 5), followed by the addition of phenylsilane under a blanket of Ar (2.0 mol%, 34 μL, 0.27 mmol, measured with a 50 μL syringe). The solution turns a bright red-orange color (Figure 1), and the reaction is monitored by thin layer chromatography (TLC) (Note 6).
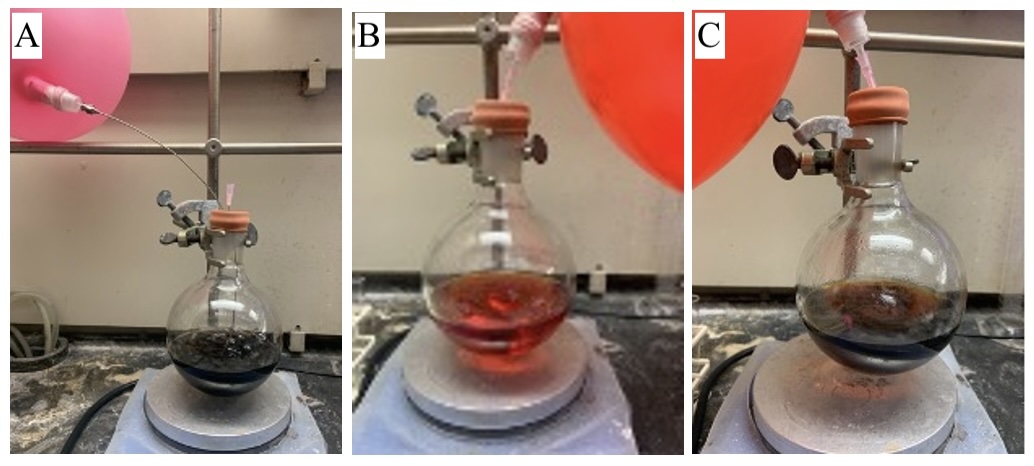
Figure 1. A) Reaction mixture before adding phenylsilane, during degassing; B) Reaction mixture immediately after addition of phenylsilane; C) Reaction mixture after 5 h of stirring (photos provided by authors)
After 5 h, no starting material is detected by TLC, at which time the reaction is concentrated directly in vacuo using a rotary evaporator (Note 7) and purified by flash column chromatography (FCC) on silica (Note 8) with approximately 600 mL of 5% ethyl acetate/hexanes (Notes 9, 10 and 11) to obtain 2.9 g (13.2 mmol, 97%) of the product as a pale-yellow oil (Notes 12, 13, and 14).
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
Co(III)salenCl,
phenylsilane,
(-)-caryophyllene oxide,
benzene,
(-)-humulene oxide II, silica,
ethyl acetate, and
hexanes.
Benzene should only be handled in a well-ventilated fume hood. Long term exposure to high levels of
benzene has been linked to cancer in humans.
2.
Benzene (>99%) was purchased from Sigma-Aldrich and used directly from the bulk, previously opened 4L container without drying or distillation.
3.
(-)-Caryophyllene oxide (95%) was purchased from Acros Organics and used as received (white solid, mp 55 - 62 ℃).
4.
(R,R)-(-)-Co(salentBu,tBu)Cl was prepared according to the procedure reported by Ford
et al.2 A 1000 mL flask equipped with magnetic stir bar was charged with (
R,
R)-(-)-Co(salen
tBu,tBu) (7 g),
DCM (580 mL), and
TsOH•H2O (2.2 g). The mixture was stirred at 20 ℃ under air for 16 h. The mixture was washed with saturated, aqueous
NaCl (500 mL x 3). The
dichloromethane layer was separated, dried over
Na2SO4 and filtered through celite (200 mL) into a round-bottomed flask. The solvent was removed by rotary evaporation (30 ℃, 700-550 mmHg). The resulting solid residue was suspended in
pentane (500 mL), collected by filtration through filter paper, and rinsed with
pentane (1 L). The solid was transferred by spatula to a round-bottomed flask, subjected to vacuum (2 mmHg) for 1 h to remove
pentane, which yielded 7 g of dark green-black solid which was used in the isomerization reaction without further purification. Checkers prepared (
S,
S)-(+)-Co(salen
tBu,tBu)Cl and observed no difference in reactivity for the MHAT procedure.
5. Failure to rigorously degas the vessel resulted in failure of the reaction. On large scale, a full balloon of
argon was used to degas the reaction mixture using a thick outlet needle (16 gauge). The balloon volume was exhausted in 20 min.
6. To separate the starting material and product for visualization by TLC, silver impregnated TLC plates were prepared. TLC plates were dipped into a 10% w/v solution of
AgNO3 in
MeCN (1 g of
AgNO3 dissolved in 10 mL of
MeCN) and dried in a 240 ℃ oven to ensure complete evaporation of the
MeCN (which required about 10 min). The plates were used with 10%
ethyl acetate in
hexanes as the eluent and stained with anisaldehyde (Figure 2).
Figure 2. Product (left), mixture of product and starting material (middle), and starting material (right) on a silver impregnated TLC plate (photo provided by authors)
7.
Benzene should be removed via rotary evaporation in a well-ventilated fume hood to prevent inhalation of vapors. During rotary evaporation, the flask was heated via water bath set to 30 ℃ and the vacuum was pulling at approximately 150 mmHg.
8. Silica gel (230-400 mesh, grade 60) was purchased from Fisher Scientific and used as received.
9.
Hexanes (>98.5%) was purchased from Fisher Scientific and used as received.
10.
Ethyl acetate (>99.5%) was purchased from Fisher Scientific and used as received.
11. A silica gel slurry is made from
hexanes and roughly 60 g of silica (
Note 8) to pack the column (internal diameter 2 inches). The crude mixture is loaded as a neat liquid. Fractions of 30 mL (5%
ethyl acetate/
hexanes) are collected. The product, identified by TLC analysis and staining with anisaldehyde, is found in fractions 7-15, which are collected in a round-bottomed flask using
DCM to rinse the fractions into the flask, and concentrated in vacuo using a rotary evaporator under reduced pressure (40 ℃, 420 mmHg).
12.
(-)-Humulene oxide II (
2) exhibits the following properties: TLC (
hexane/
ethyl acetate = 19/1 (v/v)), R
f = 0.30, stains dark purple with anisaldehyde;
1H NMR
pdf (400 MHz, CDCl
3) δ: 5.28 (ddd,
J = 15.3, 9.9, 5.2 Hz, 1H), 5.15 (d,
J = 15.8 Hz, 1H), 5.03-4.95 (m, 1H), 2.62-2.49 (m, 2H), 2.29-2.05 (m, 3H), 2.00 (dd,
J = 13.7, 9.2 Hz, 1H), 1.87 (dd,
J = 13.7, 5.6 Hz, 1H), 1.64 (dd,
J = 12.4, 9.9 Hz, 1H), 1.56 (s, 3H), 1.42-1.33 (m, 1H), 1.30 (s, 3H), 1.11 (s, 3H), 1.08 (s, 3H);
13C NMR
pdf (101 MHz, CDCl
3) δ: 143.3, 132.1, 125.9, 122.2, 63.4, 62.1, 42.7, 40.4, 36.8, 36.7, 24.9, 17.4, 15.2. GC (GC/MSD; HP-5MS UI; 139 KPa; flow rate 2 mL/min; inlet temperature 250 ℃; column temperature 50 ℃ for 0 min, then 20 ℃/min to 280 ℃, then hold 2 min): tR = 7.365 min. IR (
NaCl, thin film): 2979, 1461, 1385, 1363, 1311, 1276, 1237, 1218, 1182, 1104, 1073, 971, 915, 861, 820, 783 cm
-1; [α]
D23 -91.2° (
c = 1.0, CHCl
3); HRMS (ESI-TOF):
m/z calcd for C
15H
25O [M+H]
+: 221.1900. Found: 221.1893.
13. The purity of the compound was calculated to be >98% by qNMR
pdf using
1,3,5-trimethoxybenzene as an internal standard (
Note 15).
14. A second run on the same scale gave 2.9 g (97% yield) with >98% purity.
15.
1,3,5-Trimethoxybenzene (99%) was purchased from Sigma Aldrich and used as received.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The importance of alkene isomerization to the global chemical industry cannot be overstated. Alkene isomerization converts abundant unsaturated feedstocks into high-demand isomers, as in the reverse-Phillips process by ABB Lummus Global. This large-scale operation isomerizes 1-butene to 2-butene in a fixed-bed reactor that also catalyzes olefin metathesis between 2-butene and ethene to yield propene.
3 This abundant unsaturated petrochemical, an α-olefin,
4 also can be also converted into higher order alkenes by protonation/ rearrangement/ deprotonation sequences. Formation of the unstable carbocation intermediate, however, requires a large activation energy and strong Brønsted acid.
5 Substrates with acid-sensitive functional groups like epoxides, tertiary alcohols or silyl ethers necessitate reagents other than proton-donors, which tend to react preferentially with Lewis bases stronger than alkenes. Methods that involve allylic hydrogen atom abstraction or transition metal π-complexation can isomerize alkenes with high chemoselectivity,
6 but substrate scopes are often restricted to conjugated or mono-substituted alkenes.
6In contrast, metal hydride atom transfer (MHAT) reactions tend to react alkenes selectively at ambient temperature and tolerate a variety of functional groups that are untenable in other methods.
7, 8 Unlike Brønsted acids that react alkenes to form transient and high energy carbocation/ anion pairs,
5 metal hydrides react with alkenes via MHAT to form lower energy carbon-centered radical / metalloradical solvent cage pairs.
9 Among some MHAT isomerization reactions,
10 the initially-formed metalloradical does not appear to immediately abstract the β-hydrido radical via pseudo-unimolecular, retro-MHAT within the solvent cage. Instead, cage escape occurs quickly and the metalloradical must reencounter the carbon radical in a second, bimolecular reaction.
11As a result of its chemoselectivity and unusually broad scope, simple MHAT reactions
8 like hydration, hydrogenation and isomerization tend to exhibit high chemofidelity: they are reliable.
12 A good example of the structural complexity tolerated by MHAT is the isomerization (or retrocycloisomerization) of (-)-caryophyllene oxide (
1) to (-)-humulene oxide II (
2).
10 The product is a sesquiterpene that occurs in a variety of herbs and spices, including hops used in the brewing of beer,
13 and
Cannibas sativa.
14 While
2 might serve as an ingestion biomarker and flavor/fragrance additive to commercial materials, its availability is limited. In contrast, (-)-caryophyllene oxide is inexpensive and widely available on large scale (e.g. $310/kg from SigmaAldrich). We demonstrated an unusually efficient conversion of (-)-caryophyllene oxide to (-)-humulene oxide II using MHAT catalysis that benefits from high yield, low catalyst loading (1 mol%) and low reductant loading (2 mol%). The original procedure was trialed for Organic Syntheses with no major changes and has already been implemented successfully by the community due to the use of
2 as a building block for synthesis
15 and a scaffold for library construction.
16, 17
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Benzene; (71-43-2)
(-)-Caryophyllene oxide; (1139-30-6)
Co(III)salenCl: (R,R) Co(salen, tBu, tBu)Cl; (376652-60-7)
Phenylsilane; (694-53-1)
Argon; (7440-37-1)
Ethyl acetate; (141-78-6)
Hexanes; (110-54-3)
Silica gel; (63231-67-4)

|
Eleanor Landwehr earned her B.S. in chemical engineering from UW-Madison (USA) in 2020 where she conducted research in the lab of Jennifer Schomaker. She is currently a third-year student in the Skaggs Graduate School of Chemical and Biological Sciences at Scripps Research (USA). Her Ph.D. research has focused on accessing Class III GB alkaloids and related analogs. |

|
Ryan Shenvi earned a B.S. with honors and distinction in chemistry from Penn State University (USA) in 2003. He earned his Ph.D. at the Scripps Research Institute in 2008 as a NDSEG predoctoral fellow with Phil Baran and undertook postdoctoral studies at Harvard University as a Ruth Kirschstein postdoctoral fellow with E. J. Corey. The Shenvi lab develops new chemical reactions to navigate natural product space and interrogates mechanisms at the chemical and biological levels. |

|
Emily Chen earned her B.E. in chemical engineering from Stony Brook University in 2020 where she conducted research in the labs of Drs. Nancy Goroff and Melanie Chiu. Currently, Emily is a graduate student at the California Institute of Technology in the lab of Professor Sarah Reisman, focusing on the development of Ni-catalyzed cross-coupling reactions. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved