Org. Synth. 2024, 101, 274-294
DOI: 10.15227/orgsyn.101.0274
Synthesis of S,S-Di(pyridin-2-yl)carbonodithioate (DPDTC) for the Reduction of Carboxylic Acids
Submitted by Karthik S. Iyer,
1 Jordan R. Yirak,
1 Hubert Muchalski,*
2 and Bruce H. Lipshutz*
1Checked by Anna A. M. Miller, Daniel Rozsar and Darren J. Dixon
1. Procedure (Note 1)
A. S,S-Di(pyridin-2-yl)carbonodithioate (2; DPDTC). An oven-dried 500-mL, three-necked, round-bottomed flask, equipped with a 4-cm Teflon-coated magnetic stir bar, an argon/vacuum manifold, a thermometer, and a rubber septum (Note 2), is charged with 2-mercaptopyridine (1, 11.1 g, 100 mmol, 2.0 equiv) (Note 3). The flask is evacuated (20 mmHg, 2 minutes) and backfilled with argon three times using the manifold. Dichloromethane (150 mL) is added using a 30 mL glass syringe, followed by the addition of triethylamine (14.6 mL, 105 mmol, 2.1 equiv), using a 20 mL plastic syringe (Note 4) (Figure 1).
Figure 1. (A) Reaction set-up; (B) After addition of 2-mercaptopyridine; (C) Reaction appearance after addition of CH2Cl2 and Et3N (photos provided by authors)
The mixture is cooled to an internal temperature between 0-5 ℃ in an ice bath and triphosgene (5.40 g, 18.20 mmol, 0.36 equiv) (Note 4) is added in small portions (approximately 0.7 g each) over a period of 45 min while maintaining internal temperature of 0-5 ℃ and a positive pressure of argon (Note 5) (Figure 2).
Figure 2. (A) Cooling the reaction in an ice-bath; (B) Addition of triphosgene; (C) Reaction appearance after complete addition of triphosgene (photos provided by authors)
The reaction is allowed to warm to 22 ℃ and stirred for 16 h. Triethylammonium chloride is observed to precipitate as the reaction proceeds to completion (Figure 3A). The rubber septum is removed, water (100 mL) is added, and the mixture is stirred under a steady stream of argon for 5 min. The reaction mixture is transferred to a 500 mL separatory funnel, and the aqueous phase is separated. The organic layer is washed with an additional portion of water (100 mL) and the combined aqueous layers are extracted with dichloromethane (2 x 25 mL). The combined organic layers are washed with saturated aq NaCl (50 mL) and then dried over anhydrous Na2SO4 (24 g) (Note 6). The solution is vacuum filtered (200 mmHg) through a medium porosity sintered glass funnel into a 500 mL round-bottomed flask and concentrated by rotary evaporation (40 ℃, 200 mmHg) until 30 mL of volume remains. The residue is transferred into a 100 mL round-bottomed flask and concentrated further (40 ℃, 15 mmHg) to yield a brown oil (Figure 3C). This oil is then placed in a refrigerator (0 ℃, 60 min) to initiate crystal formation (Note 7).
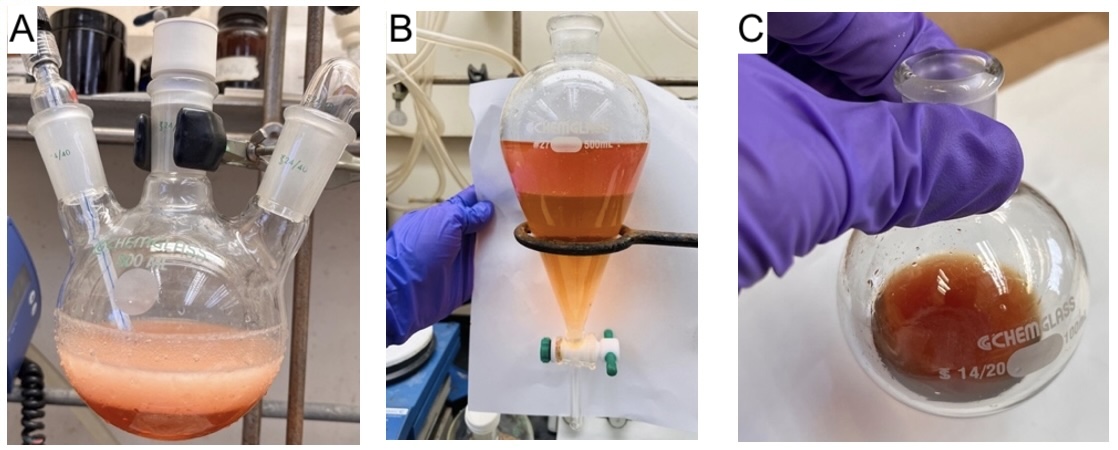
Figure 3. (A) Appearance of the reaction after 16 h; (B) Layer separation in a 500 mL separatory funnel; (C) Brown oil (crude DPDTC) after concentration (photos provided by authors)
The flask with the crystals is placed under high vacuum (20 ℃, 7.5 mm Hg) for 90 min to yield 2 as a brown crystalline solid (12.2 g) (Figure 4A). The solid is crushed with a spatula and a cold (0 ℃) mixture of pentane and diethyl ether (1:1, v/v, 50 mL) (Figure 4B) is added to wash the product, and the solid is vacuum filtered (200 mm Hg) through a medium porosity sintered glass funnel into a 250 mL round-bottomed flask. The solid is then washed with an additional portion of chilled (0 ℃) mixture of pentane and diethyl ether (1:1, v/v, 50 mL) while agitating the solid on the funnel. The solid is then dried on the funnel under vacuum (20 mm Hg) for 5 min under a constant flow of nitrogen gas (Figure 4C) to give 11.1 g (90%) of a light reddish brown to light yellow solid (Notes 8, 9, 10, 11, 12, 13, and 14) (Figure 4C).

Figure 4. (A) Appearance of crude DPDTC after crystallization of the oil; (B) Trituration with 1 : 1 diethyl ether/pentane; (C) Set-up to dry DPDTC under constant flow of nitrogen (D) pure DPDTC after drying (photos A, B, and D provided by authors)
B. 5-(2,5-Dimethylphenoxy)-2,2-dimethylpentan-1-ol (4). An oven dried 250-mL round-bottomed flask equipped with a 3-cm Teflon-coated magnetic stir bar is charged with Gemfibrozil (3; 2.50 g, 10.00 mmol, 1 equiv), N,N-dimethylaminopyridine (122 mg, 1.00 mmol, 0.10 equiv), and DPDTC (2; 2.72 g, 10.95 mmol, 1.10 equiv). The flask is covered with a rubber septum equipped with a vent needle (Note 15) (Figure 5A) and is stirred neat at 65 ℃ in an oil bath for 1 h (Note 16). The reaction mixture quickly turns into a viscous brown liquid upon heating to 65 ℃ (Figure 5B).
Figure 5. (A) Reaction set-up with all the starting materials; (B) Reaction appearance at the start (65 ℃, clear oil); (C) Reaction appearance after the formation of the thioester and cooling to 22 ℃ (yellow precipitate) (photos provided by authors)
The reaction is allowed to cool to 22 ℃, upon which the mixture solidifies and turns dark yellow (Figure 5C). The rubber septum is removed, 95% EtOH/H2O (20 mL, 0.5 M) is added, and the solution is cooled to an internal temperature between 0-5 ℃ using an ice-bath while maintaining constant stirring (Figure 6A). Sodium borohydride (1.14 g, 30.00 mmol, 3.00 equiv) is added in small portions (Note 17) over 20 min, and the reaction is stirred for additional 10 min while cooled in an ice bath (Figure 6 B, C). The flask is sealed with a rubber septum equipped with a vent needle to avoid over pressurization due to hydrogen formation, then the ice-bath is removed, and the reaction is allowed to warm to 22 ℃ while stirring for 30 min (Figure 6D).

Figure 6. (A) Reaction appearance after addition of 95% EtOH/H2O; (B) Addition of NaBH4 portion wise; (C) Reaction appearance after addition of NaBH4 (notice foaming due to evolution of H2 gas) (D) Removal of the ice-bath and employing a vent needle for outflow of H2 (photos provided by authors)
Upon completion, as monitored by TLC (Note 18), the reaction mixture is concentrated by rotary evaporation (40 ℃, 75 mm Hg) to remove most of the EtOH. To the residue is added water (50 mL) and ethyl acetate (100 mL), and the mixture is stirred for 5 min and then transferred into a 250-mL separatory funnel. The organic layer is separated, and the aqueous layer is extracted with EtOAc (2 x 50 mL). The combined organic layers are dried over anhydrous Na2SO4 (24 g). The solution is filtered through a medium porosity sintered glass funnel into a 250 mL round-bottomed flask and concentrated by rotary evaporation (40 ℃, 75 mm Hg), to afford crude 4 as a yellow oily solid. The crude product is purified by flash chromatography (Note 19) using EtOAc and hexanes as the eluent to afford compound 4 as a pale-yellow oil (2.23 g, 94%) (Notes 20, 21, and 22) (Figure 7).
Figure 7. Final product 4 after column chromatography (Note 19) (photos provided by authors)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out regarding each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/about/governance/committees/chemical-safety.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
2-mercaptopyridine,
dichloromethane,
triethylamine,
triphosgene,
NaCl, anhydrous
Na2SO4,
pentane,
diethyl ether,
Gemfibrozil,
N,N-dimethylaminopyridine,
ethanol,
sodium borohydride,
ethyl acetate, hexanes,
1,3,5-trimethoxybenzene,
KMnO4, and
CDCl3. The solid reagent
triphosgene is a less hazardous substitute for highly toxic gaseous phosgene, however, it should be handled very carefully.
Triphosgene may be fatal if inhaled and causes burns by all exposure routes. This water-reactive substance liberates toxic gas (phosgene and HCl) upon exposure to water.
Triphosgene is a lachrymator and can decompose violently at elevated temperatures.
Triphosgene should be weighed out and the reaction should be performed in a well-ventilated fume hood.
2. The apparatus is flame-dried and maintained under an atmosphere of argon or
nitrogen during the reaction.
3. The color and water content in
2-mercaptopyridine depends on the source from which it is obtained (Figure 8). It was therefore dried overnight under high vacuum (30 ℃, 1 mm Hg) to remove any adventitious water, before using it further.
2-Mercaptopyridine was obtained from Sigma Aldrich and Chem-Impex and was dried before use.
Figure 8. Variation in appearance of 2-mercaptopyridine obtained from different vendors (photo provided by authors)
4. Reagents and solvents used in this preparation are commercially available and used without further purification.
Triphosgene (>98%) was purchased from TCI chemicals and the checkers purchased from Fluorochem (>99%).
Triethylamine (>99.5%) was purchased from Sigma Aldrich.
Dichloromethane was obtained from Fisher Scientific and the checkers purchased from Sigma Aldrich (HPLC grade) and was stored under 4 Å molecular sieves for 16 h before using in the reaction.
Gemfibrozil was obtained from Combi Blocks Inc.
Sodium borohydride was purchased from Sigma Aldrich.
Ethanol (95%; 190 proof) was obtained from Gold Shield Dist. Inc and the checkers purchased absolute
ethanol (99.8%) from Sigma Aldrich and diluted it with deionized water (Merck Millipore reverse osmosis purification system) to make a 95% solution.
5. Each addition of
triphosgene will gradually increase the temperature of the reaction mixture. Stirring was continued and the mixture was allowed to cool back to 0-2 ℃ before the next addition.
6. TLC analysis (Figure 9) of the organic phase after aqueous work-up.
Figure 9. TLC of organic phase at the end after aqueous workup before trituration showing reaction mixture (R, left, Rf = 0.19) vs starting material 2-mercaptopyridine (SM, right) with a central co-spot (CC) using 30% EtOAc/hexanes as the eluent; (A) Visualized under 254 nm UV lamp; (B) The same TLC after staining with standard KMnO4 stain. The spots appear upon slight heating using a heat gun (photos provided by authors)
7. Upon standing at 10 ℃ or below, the oil slowly starts to solidify to a crystalline solid.
8. TLC analysis (Figure 10) of the solid after trituration.
Figure 10. TLC of organic phase at the end after trituration showing solid DPDTC (DP, left, Rf = 0.19) vs starting material 2-mercaptopyridine (2-Py-SH, right) with a central co-spot (CO) using 30% EtOAc/hexanes as the eluent; (A) Visualized under 254 nm UV lamp; (B) The same TLC after staining with standard KMnO4 stain. The spots appear upon slight heating using a heat gun (photos provided by authors)
9. Characterization data for
S,S-di(pyridin-2-yl) carbonodithioate (
DPDTC) (
2). TLC conditions: 30%
EtOAc/hexanes, R
f = 0.19 (silica gel plate, 254 nm UV, yellow spot using
KMnO4 stain).
1H NMR
pdf (400 MHz,
CDCl3) δ: 8.63 (d,
J = 4.9 Hz, 2H), 7.79 - 7.66 (m, 4H), 7.34 - 7.31 (m, 2H);
13C NMR
pdf (126 MHz,
CDCl3) δ: 185.6, 150.6, 150.6, 137.7, 130.6, 124.3; IR (film): 3046, 1712, 1656, 1572, 1420, 1083 cm
-1; HRMS (ESI)
m/z: calcd. for C
11H
8N
2OS
2 249.0151, found 249.0146; mp: 38-40 ℃.
10. The purity of the compound was determined to be 98.5% by qNMR
pdf using
1,3,5-trimethoxybenzene (>95% purity purchased from Fluorochem) as an internal standard.
11. A second reaction on identical scale provided 10.8 g (87%) of the product with purity of 97.4%.
12. It should be noted that the product of this reaction has limited stability at room temperature, particularly if exposed to air, and visible yellowing along with the appearance new peaks in the
1H NMR spectrum are observed due to the hydrolysis back to
2-mercaptopyridine, if it is improperly stored. The authors report that it can be kept under inert atmosphere at 4 ℃ for at least several months without decomposition. The checkers note that on storage for several weeks residual moisture can cause decomposition resulting in the evolution of gas and pressure build up in the vessel.
13. The authors report that the color of
DPDTC (
2) can range from light reddish brown to yellow (Figure 11), depending on the level of impurities and the source of
2-mercaptopyridine.
Figure 11. Variability in color in different batches of DPDTC after trituration (photos provided by authors)
14. Owing to an academic scale setup and the volatility of
methylene chloride, only 40% of the solvent was recovered. However, in an industrial setup, greater efficiency could be achieved in recovering the
methylene chloride. Moreover, this protocol could also be carried out in
acetone, as previously reported by the authors.
8d However, after the reaction, a solvent swap to
methylene chloride or
ethyl acetate needs to be carried out before the aqueous workup. Additionally, in this protocol, the major portion of the extraction solvent comes from the solvent used in the reaction.
15. A vent needle was employed to facilitate the escape of carbon dioxide generated in the process, and to avoid pressure build-up.
16. TLC conditions for thioester formation (step 1): 10%
EtOAc/hexanes, R
f = 0.24 (silica gel plate, 254 nm UV, dark blue spot using
PMA stain).
Figure 12. (A) TLC of thioesterification of gemfibrozil showing the reaction mixture (R, left) vs starting material gemfibrozil (acid, right), and DPDTC (DP, rightmost) with a central co-spot (CO) using 5% EtOAc/hexanes, as the eluent. The plate was run twice and visualized under 254 nm UV lamp, clearly showing the disappearance of the acid; (B) Using 10% EtOAc/hexanes as the eluent; (B) The same TLC (B) after staining with standard phosphomolybdic acid (PMA) stain. The spots appear upon slight heating using a heat gun. The acid stains red whereas the thioester stains dark blue (photos provided by authors)
17. Evolution of
H2 gas was observed while adding
NaBH4. Use of an ice bath to maintain a temperature between 0-5 ℃ and adding
NaBH4 portion wise is recommended to prevent thermal runaway.
18. TLC analysis of alcohol
4: 10%
EtOAc/hexanes, R
f = 0.16 (silica gel plate, 254 nm UV, red spot using
PMA stain)
Figure 13. (A) TLC of reduction showing the reaction mixture (R2, left) vs thioester of Gemfibrozil (R1, right), with a central co-spot (Co) using 10% EtOAc/hexanes, as the eluent. The plate was visualized under 254 nm UV lamp; (B) The same TLC after staining with standard phosphomolybdic acid (PMA) stain. The spots appear upon slight heating using a heat gun. The thioester stains blue whereas the alcohol stains red, clearly showing the disappearance of the thioester intermediate (photos provided by authors)
19. Column chromatography was carried out as follows: A flash column with 3-cm outer diameter and a capacity of 800 mL was charged with silica gel (Silicycle Siliaflash P60 particle size 0.040-0.063 mm purchased from Silicycle; used as received), the checkers purchased silica gel from Sigma Aldrich (technical grade, pore size 60 Å, 230-400 mesh particle size, 40-63 μm particle size) using a wet-pack method (60 g of silica was primed using 200 mL of hexanes). This gave a silica bed a height of 9.5 inches (23 cm). The crude mixture was adsorbed onto 4-6 g of silica and was dry loaded onto the silica using a powder funnel. Sand was then added to fill 2 cm above the silica. Hexanes (150 mL) was used initially, followed by 10%
EtOAc/hexanes (250 mL), 12.5%
EtOAc/hexanes (250 mL), and 15%
EtOAc/hexanes (250 mL). A total of 40 fractions (25 mL each) was collected with fractions 11-28 containing the product. All the fractions were confirmed by TLC and then combined and concentrated using rotary evaporation (40 ℃, 70 mmHg) to afford
2 as a colorless oil. This oil was transferred into a 6-dram vial (20 mL capacity) and dried overnight under high vacuum (25 ℃, 7 mmHg) to afford
4.
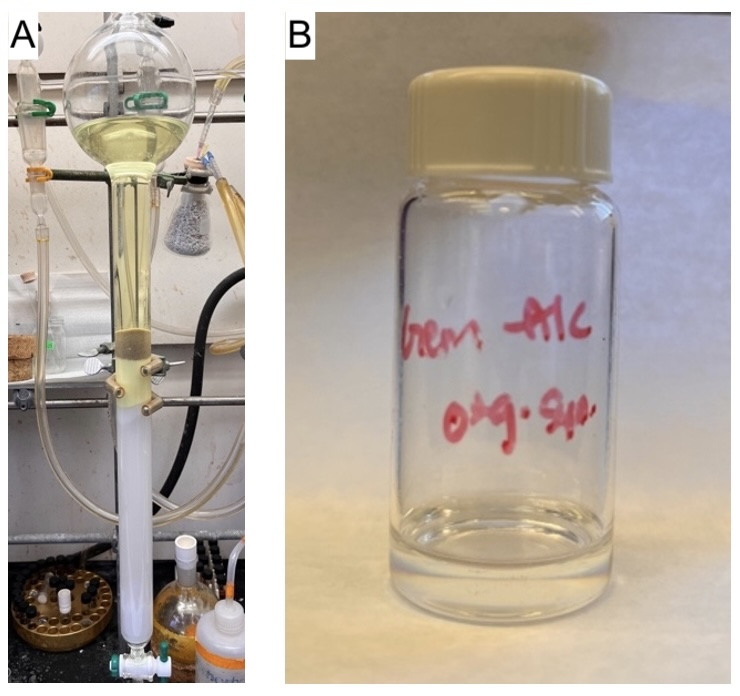
Figure 14. (A) Column used for flash chromatography; (B) Appearance of the product alcohol 2 after column chromatography (photos provided by authors)
20.
Characterization data for 5-(2,5-dimethylphenoxy)-2,2-dimethylpentan-1-ol (4). 1H NMR
pdf (400 MHz,
CDCl3) δ: 7.01 (dd,
J = 7.4, 0.9 Hz, 1H), 6.66 (dd,
J = 7.9, 1H), 6.63 (s, 1H), 3.93 (t,
J = 6.4 Hz, 2H), 3.37 (d,
J = 5.5 Hz, 2H), 2.31 (m, 3H), 2.18 (s, 3H), 1.84 - 1.72 (m, 2H), 1.48 - 1.31 (m, 3H), 0.93 (s, 6H);
13C NMR
pdf (101 MHz,
CDCl3) δ: δ 157.2, 136.6, 130.4, 123.7, 120.8, 112.2, 72.0, 68.7, 35.1, 34.9, 24.3, 24.0, 21.6, 15.9. IR (film): 3387, 2953, 1616, 1585, 1509, 1266 cm
-1. HRMS (GC-EI
+)
m/z: [M + Na]
+ calcd for C
15H
24O
2Na 259.1669, found 259.1680. The authors report that the product can be stored at rt without decomposition.
21. Purity of the compound was determined to be 97.9% by qNMR
pdf using
1,3,5-trimethoxybenzene (>95% purity purchased from Fluorochem) as an internal standard.
22. A second reaction on identical scale provided 2.32 g (98%) of the product with purity of 97.3%.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Almost every introductory organic chemistry textbook discusses reductions of carboxylic acids and their derivatives, such as esters, acid halides, and (mixed) anhydrides, with the emphasis on hydride-containing reducing agents, most notably lithium aluminum hydride (LAH) and diisobutylaluminum hydride (DIBAL-H).
3 While their contribution to organic synthesis is secure, they require appropriate technical expertise to carry out the reactions and their propensity to react with air and moisture, as well as reactivity/selectivity concerns, are well-known drawbacks. Additionally, as seen today through a sustainability lens, there is plenty of potential for methods that, although similarly successful, are both more functional group tolerant and contemporary, i.e., where the total environmental impact is reduced.
The direct reduction of carboxylic acids to alcohols is a rather challenging, even dangerous transformation, especially at scale using LAH or DIBAL-H.
4,5,6,7 To make these four electron reductions greener, a new technology was envisioned that avoids overly reactive hydride-based reagents, takes place very efficiently in a green reaction medium, and is very tolerant of the functional groups present in the starting acid. The approach developed is based on the use of
S,S-di(pyridin-2-yl) carbonodithioate (DPDTC;
2),
8a,8b,8c,8d,8e which converts carboxylic acids to their corresponding
S-2-pyridyl thioesters that can be used
in-
situ. Their exposure to sodium borohydride in a one-pot fashion in 95% EtOH/H
2O at 22 ℃ directly affords the targeted alcohols.
In this context we investigated the scope of reductions of carboxylic acids to alcohols.
9 As summarized in Table 1, a wide range of functionality present in both aliphatic and aromatic carboxylic acids is reduced in good-to-excellent yields, including various heteroaromatic carboxylic acids. To further test generality, some late-stage functionalized substrates from the Merck Informer Library,
10 as well as related bioactive molecules, were also screened to afford the targeted alcohols in good yields.
Overall, an environmentally responsible method for reduction of carboxylic acids to alcohols has been developed, utilizing a recyclable aqueous reaction medium. Unlike prior reports based on harsh hydride-containing reagents such as LAH and DIBAL-H, this technology allows for a broad selection of substrate types, including functionalized educts, suggesting its potential applications to late-stage functionalization of value in medicinal chemistry.
Table 1. Substrate scope for carboxylic acid reduction.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
2-Mercaptopyridine (1) (2637-34-5)
Triphosgene (32315-10-9)
Triethylamine (121-44-8)
2,2-Dimethyl-5-(2,5-dimethylphenoxy)pentanoic acid, 2,2-dimethyl-5-(2,5-xylyloxy)valeric acid, 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoic acid (Gemfibrozil) (3) (25812-30-0)
Sodium borohydride (16940-66-2)

|
Karthik Iyer received his Bachelor of Technology (B. Tech) degree in Pharmaceuticals chemistry and Technology from the Institute of Chemical Technology, Mumbai, India in 2020. He is currently a fourth-year graduate student in the laboratory of Bruce Lipshutz at the University of California, Santa Barbara. |

|
Jordan Yirak is a third-year undergraduate student, pursuing his B. S. in Chemistry at the University of California, Santa Barbara. He is currently an undergraduate researcher in the laboratory of Bruce Lipshutz at the University of California, Santa Barbara. |

|
Hubert Muchalski completed his undergraduate studies in chemistry at the Wrocław University of Science and Technology, Poland. He obtained his Ph.D. from Vanderbilt University in 2012 under the supervision of Dr. Jeffrey N. Johnston. Following a postdoctoral appointment with Dr. Ned A. Porter, he joined the faculty at California State University as an Assistant Professor and was granted tenure as an Associate Professor in 2021. |

|
Bruce H. Lipshutz was born in the Washington Heights section of New York City in December 1951. He initially studied organic chemistry under Howard Alper, with whom he did undergraduate research at SUNY at Binghamton (B.A. 1973). His Ph.D. was taken from Yale (1977) under the direction of Harry Wasserman. He was then an American Cancer Society postdoctoral fellow with E.J. Corey. In 1979 he joined the faculty at UCSB as an Assistant Professor and became Full Professor in 1987. |

|
Anna Miller completed her integrated masters degree (MChem) in chemistry at the University of Glasgow, Scotland, with an industrial placement year in process chemistry at Pfizer. She is now a third year DPhil student under the supervision of Prof. Darren J. Dixon at the University of Oxford studying total synthesis of complex natural products. |

|
Daniel Rozsar obtained his BSc and MSc in chemistry at the University of Szeged, Hungary. He recently completed his DPhil at the University of Oxford, where he studied enantioselective organosuperbase catalysis under the supervision of Prof. Darren J. Dixon. In 2022 he spent one year at The University of Tokyo in Prof. Motomu Kanai's lab, supported by JSPS. Daniel will soon be joining Prof. Alexander Radosevich's group at MIT as a postdoctoral associate. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved