Org. Synth. 2024, 101, 382-394
DOI: 10.15227/orgsyn.101.0382
α-Arylation of N-Boc Pyrrolidine
Kevin Campos,*
1 Melissa Cummings, Hengyu Li, and Feng Peng
Checked by Preston Mott and Sarah E. Reisman
1. Procedure (Note 1)
A. tert-Butyl (S)-2-(4-(methoxycarbonyl)phenyl)pyrrolidine-1-carboxylate. An oven dried 500 mL three-necked round-bottomed flask is attached to a Schlenk line with N2 as inert gas and equipped with a temperature probe, a Teflon-coated magnetic stir bar (2 x 0.8 cm), and a rubber septa. The flask is then degassed with nitrogen via five evacuate-refill cycles. MTBE (120 mL), N-Boc-pyrrolidine (1) (10.0 g, 58.4 mmol, 1.20 equiv), and (+)-sparteine (13.7 g, 58.4 mmol, 1.20 equiv) are charged into the flask, and the solution is cooled to between -70 °C to -78 °C over 30 min with a dry ice/acetone bath (Note 2). 1.4 M sec-BuLi in cyclohexane (45.2 mL, 63.3 mmol, 1.30 equiv) is then added dropwise over 30 minutes via syringe into the yellow solution while maintaining the internal temperature below -65 °C (Note 3). The resulting orange-red solution is aged at -65 °C to -78 °C for 3 h (Figure 1 and Note 4). A 0.7 M solution of ZnCl2 in THF (97.3 mL, 68.1 mmol, 1.40 equiv) is then added dropwise over 60 min via a syringe while the internal temperature is kept below -65 °C (Note 5). Once the addition is complete, the dry ice/acetone bath is removed and exchanged for a water bath at ~25 °C (Figure 2). As the internal temperature rises, the slurry becomes yellow. After reaching room temperature (~25 °C), the reaction is aged for an additional 45 min, and the slurry thins into a hazy yellow solution.
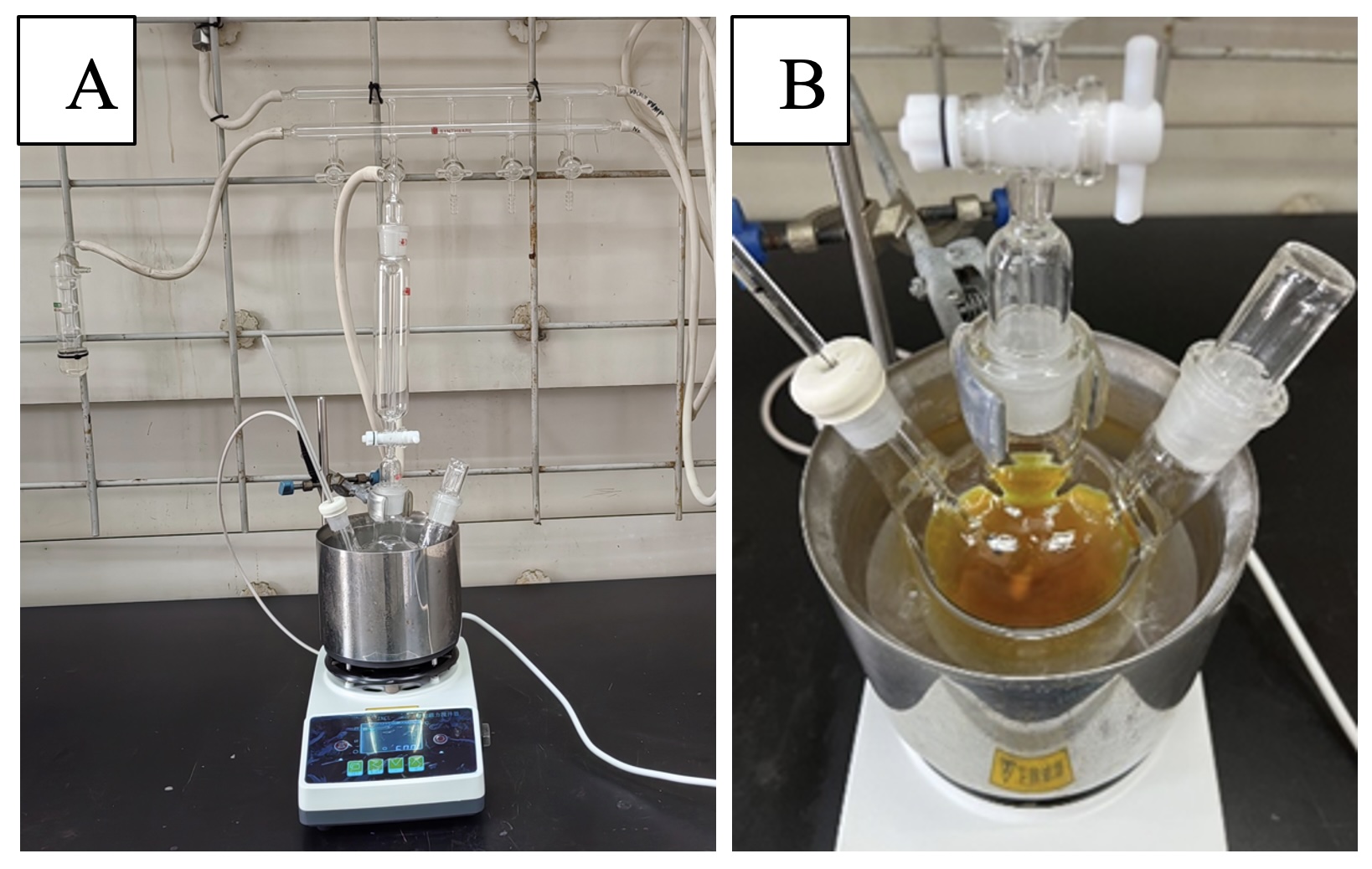
Figure 1. A) Reaction setup; B) Reaction mixture stirred with s-BuLi in dry ice/acetone bath (photos provided by authors)
Figure 2. A) Reaction mixture after addition of ZnCl2; B) Reaction mixture stirred at room temperature (photos provided by authors)
Methyl 4-bromobenzoate (10.5 g, 48.8 mmol, 1.00 equiv) is then charged as a solid in a single portion (Note 6). The slurry is stirred for 15 min at room temperature to allow for dissolution before simultaneously charging solid tBu3P·HBF4 (0.85 g, 2.92 mmol, 0.06 equiv) and Pd(OAc)2 (0.54 g, 2.43 mmol, 0.05 equiv) into the reaction. The reaction mixture is degassed with nitrogen via three evacuate-refill cycles (medium vacuum). An exotherm is observed within the first few minutes when a yellow/orange precipitate forms (Note 7). The color of the reaction mixture changes to red and dark green finally (Figure 3A). The reaction is then aged overnight at room temperature (Note 8).
Figure 3. A) Reaction mixture after addition of Pd(OAc)2 and tBu3P·HBF4 ; B) Reaction mixture after quenching with aq. ammonium hydroxide C) Reaction TLC, visualized at 254 nm (photos provided by authors)
The resulting tan slurry is quenched with aq. ammonium hydroxide (3.5 mL) and the suspension (Figure 3B) is stirred for 1 h (Notes 8 and 9) before being filtered with suction through celite (30 g) in a 150 mL medium porosity fritted glass filter funnel (Figure 4A) (Note 9). The filter cake is rinsed with MTBE (2 X 40 mL). The filtrate is then transferred into a 500 mL separatory funnel and washed with 1.0M aq HCl (2 X 100 mL) and water (2 X 100 mL) (Figure 4B). The resulting solution is dried with MgSO4 (30g), filtered with suction through a 150 mL medium porosity fritted glass filter funnel, and concentrated by rotary evaporation (35 °C, 20 mmHg) to obtain 2 as an orange oil.
Figure 4. A) Suspension filtered through a pad of Celite; B) Filtrate in a separatory funnel with 1 M aq. HCl (photos provided by authors)
The crude product is then purified by column chromatography [300 g silica; 30 x 5 cm]. The product is loaded onto the column with 10 mL of
dichloromethane and initially eluted with 300 mL of hexanes (discarded). Elution is continued with 750 mL 5%
EtOAc/hexanes after which point fraction collection is begun (75 mL fractions). Elution is continued with 1,000 mL of 5%
EtOAc/hexanes, 2,000 mL 10%
EtOAc/hexanes followed by 2,000 mL 20%
EtOAc/hexanes. The desired product is collected in fractions 43 to 65. The product fractions are combined and concentrated by rotary evaporation (35 °C, 20 mmHg) to provide
2 (11.1 g-11.5 g, 75%-77% yield, 91.1%-91.6% ee) as a white solid (
Note 10). The ee is then further enhanced by recrystallization. A 3-neck 100 mL flask with overhead stirrer (19 mm x 44 mm stir blade),
nitrogen inlet, and rubber septum was charged with the product (
Note 11). The product (11.1 g, 36.5 mmol) is first dissolved in
DMF (25 mL). A small amount of
H2O (1.9 mL) is then added, and the solution is seeded with 300 mg of product (
Note 12). The seedbed forms as a thin suspension and is aged for 1 h before adding additional
H2O (2.6 mL) in the first 1 h and
H2O (6.7 mL) in the next 1 h using a syringe pump (
Note 13 and Figure 5). The slurry is then agitated overnight.
The resulting tan slurry is quenched with aq. ammonium hydroxide (3.5 mL) and the suspension (Figure 3B) is stirred for 1 h (Notes 8 and 9) before being filtered with suction through celite (30 g) in a 150 mL medium porosity fritted glass filter funnel (Figure 4A) (Note 9). The filter cake is rinsed with MTBE (2 X 40 mL). The filtrate is then transferred into a 500 mL separatory funnel and washed with 1.0M aq HCl (2 X 100 mL) and water (2 X 100 mL) (Figure 4B). The resulting solution is dried with MgSO4 (30g), filtered with suction through a 150 mL medium porosity fritted glass filter funnel, and concentrated by rotary evaporation (35 °C, 20 mmHg) to obtain 2 as an orange oil.
Figure 5. A) Product in DMF; B) Suspension after the addition of seed; C) Slurry end of water charge (photos provided by checkers)
The product is filtered in a 150 mL, medium fritted glass funnel, washed with H2O (3 x 15 mL), and dried under house vacuum overnight. A white crystalline product (9.91 g - 10.1 g, 67%-68% overall yield) is obtained (89-91% yield from the crystallization) and high ee (98.8%) (Note 14) (Figure 6).
Figure 6. A) Filtration; B) Final product (photos provided by authors)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/about/governance/committees/chemical-safety.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
sec-BuLi,
ZnCl2 in
THF,
(+)-sparteine,
tBu3P·HBF4, and
Pd(OAc)2, as well as the proper procedures for the deprotonation of
N-Boc-pyrrolidine.
2. New bottles of
N-Boc-pyrrolidine (98%),
MTBE,
methyl-4-bromobenzoate (98%), and
THF were purchased from Aldrich and used directly in the reaction without further purification. The kf of the
MTBE = 60 ppm.
(+)-Sparteine (>98%) was purchased from Oakwood Chemicals with kf < 2000 ppm. The authors received
(+)-sparteine from TCI with water content > 10000 ppm. The kf was then controlled to be below 2000 ppm using dry
MTBE azeotrope distillation with rotary evaporation before the experiment.
tBu3P·HBF4 (>98%) and
Pd(OAc)2 (>98%) were purchased from TCI and used as received. The checkers used anhydrous
ZnCl2 in
THF (0.7 M) purchased from Acros Organics and used directly as received. The authors used anhydrous
ZnCl2 in
THF (1.0 M) purchased from Infinity Scientific and used as received.
3. Addition of
sec-BuLi is fairly exothermic. It is necessary to keep the internal temperature of the reaction <-65° in order to achieve high ee. 1.4 M
Sec-BuLi in
cyclohexane was purchased from Aldrich and used directly. For the titration of
sec-BuLi, the authors used Chong's method (
Journal of Organometallic Chemistry 1997,
542, 281-283).
4. A 3-hour age time appears to be necessary for complete deprotonation.
5. The orange-red solution becomes a yellow slurry with the addition of
ZnCl2. During this addition, it is also necessary to maintain an internal temperature <-65°C.
6. The charges of solids to the reaction are performed under a positive flow of
N2. The reaction is generally stirred for 15 to 30 minutes to allow
methyl-4-bromobenzoate to dissolve.
7. The checkers observed an exotherm up to 33 °C.
8. The reaction was generally very fast, with >80% conversion within the first 30 minutes. The remaining 20% took 3-4 hours to reach completion, but could be left overnight without any decomposition occurring. Reaction was monitored by quenching a sample in aq.
NH4OH and diluting with
MeCN.
9. Aq.
ammonium hydroxide was found to precipitate most of the zinc salts as filterable solids.
10. In 16%
EtOAc/Hexanes, the product as a R
f of 0.22 and the starting material has a R
f of 0.72. The checkers prepared a racemic standard by performing the reaction in the absence of
(+)-sparteine.
11. The authors used magnetic stirring for the recrystallization. The checkers used overhead stirring for the recrystallization. The checkers noted that magnetic stirring gave less enantioenriched product.
12. Seeds were prepared via the same crystallization procedure (
DMF/water) at 300 mg scale through a self-seeding process. The checkers recrystallized the seed crystals multiple times until they were >99% ee. The checkers noted that lower purity seeds gave slightly lower ee.
13. As water is added, the product crystallizes as a very voluminous, fluffy white solid.
14. The product
2 is fully characterized: mp = 79.5-81.4 °C;
1H NMR
pdf (400 MHz, CDCl
3): δ 7.98 (d,
J = 8.4 Hz, 2H), 7.24 (d,
J = 8.4 Hz, 2H), 5.05 - 4.72 (m, 1H), 3.90 (s, 3H), 3.71 - 3.45 (m, 2H), 2.45 - 2.20 (m, 1H), 2.00 - 1.71 (m, 3H), 1.44 (s, 3H), 1.16 (s, 6H);
13C NMR
pdf (100 MHz, CDCl
3, 25 °C) rotameric mixture, resonances for minor rotamer are enclosed in parenthesis (): δ 167.1-both rotamers, 154.5-both rotamers, 150.7 (149.7), (129.9) 129.7, 128.6-both rotamers, 125.6-both rotamers, 79.5-both rotamers, 61.3 (60.7), 52.1-both rotamers, (47.5) 47.2, 36.0 (34.9), (28.5) 28.2, (23.7) 23.4; FTIR (film, NaCl, cm
-1) = 2975, 2877, 1723, 1695, 1610, 1577, 1435, 1392, 1365, 1307, 1278, 1170, 1111, 1102, 1017, 967, 901, 872, 771, 708. HRMS (ESI): Calculated for C
17H
24NO
4 [M+H]
+ 306.1705; Found 306.1701. SFC: 98.8% e.e. Column: Chiracel OB-H 250 x 4.6mm, 5µm. Mobile phase: 97% CO
2 - 3% IPA (15 mins), 2.5 mL/min. λ = 230 nm, RT [(R)-
2] = 5.92 min, RT [(S)-
2] = 8.01 min. [α]
20D -108.5 (c 1.00,
acetone). The purity of
2 was determined to be >97 wt% (99.5%-99.6%) by qNMR
pdf using
1,3,5-trimethoxybenzene (Aldrich 99.9%) as the internal standard.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
α-Arylpyrrolidines represent an important structural motif in biologically active compounds and effective chiral controllers in asymmetric synthesis.
2 Examples of natural products (Figure 7) include (
S)-nicotine, which as well as being a major constituent of tobacco exhibits significant pharmacological effects on central nervous system (CNS) diseases;
3 (
R)-crispine A, which shows cytotoxic activity against SKOV3, KB and HeLa human cancer lines;
4 and (
R)-harmicine, which shows anti-leishmania activity.
5 The activity of (
S)-nicotine at nicotinic acetylcholine receptors led to the synthesis and evaluation of nicotine analog (
S)-SIB 1508Y for the treatment of Parkinson's disease.
6 In addition, α-arylated pyrrolidines are motifs in chiral auxiliaries and chiral organocatalysts.
7
Figure 7. Examples of 2-aryl pyrrolidines
Few methods exist for the synthesis of these privileged structures, and all suffer from either long synthetic sequences, low yields, lack of generality, or modest enantioselectivity.
8 To this end, we recently reported a direct method to access enantioenriched 2-arylpyrrolidines through arylation of (
R)-2-lithio-N-Boc-pyrrolidine obtained from (-)-sparteine-mediated, enantioselective deprotonation of N-Boc-pyrrolidine (
1).
9,10 The protocol involves deprotonation of N-Boc pyrrolidine using
sec-BuLi/(-)-sparteine or (+)-sparteine in MTBE at -78
oC, transmetalation with ZnCl
2 and Negishi coupling using Pd(OAc)
2,
t-Bu
3P-HBF
4 and the aryl bromide.
The procedure described here (Figure 8) is adapted from the previous report. Although (-)-sparteine is applied mostly in our previous reports, the method works equally well with commercially available (+)-sparteine. We believe this procedure will be very useful to OrgSyn readers.
Figure 8. One-pot pd-catalyzed direct arylation of N-Bocpyrrolidine
Appendix
Chemical Abstracts Nomenclature (Registry Number)
N-Boc-pyrrolidine:tert-Butyl pyrrolidine-1-carboxylate; (1) (86953-79-9)
(+)-Sparteine: Pachycarpine; (492-08-0)
(s-Butyllithium solution: Lithium, butan-2-yl;(598-30-1)
ZnCl2: Zinc chloride (ZnCl2);(7646-85-7)
Methyl-4-bromobenzoate: 4-Bromobenzoic acid methyl ester;(619-42-1)
tBu3P.HBF4: Tri-tert-butylphosphonium tetrafluoroborate;(131274-22-1)
Pd(OAc)2: Palladium acetate;(3375-31-3)
MTBE: Methyl tertary butyl ether;(1634-04-4)

|
Kevin Campos received a B.S. degree in Chemistry from Virginia Tech in 1993. In 1999, he obtained his PhD from Professor David A. Evans at Harvard University. Kevin joined the Department of Process Research at Merck in 1999, and over the last 24 years, he has established himself as a leader in both drug discovery and development of important products at Merck. He is currently Vice President and Head of Process Research and Development, responsible shepherding programs across all modalities from Discovery to Commercial Filing. Under his leadership, several commercial manufacturing routes for novel therapies have been launched, including ZEPATIER™, PREVYMIS™, PIFELTRO™, ZERBAXA™, RECARBRIO™, BRIDION™, WELIREG™, LYFNUA™, and LAGEVRIO™ and his team has been recognized by the US EPA with the Green Chemistry Challenge Award 6 years in a row. He serves as the chairman of the R&D Council of New Jersey and was recognized with the ACS Earle B. Barnes Award for Leadership in Chemical Research Management in 2022. |

|
Melissa Cummings received her B.S. in Chemistry from William and Mary in 2005, and her M.M.S. from Wake Forest School of Medicine in 2011. She worked as an associate chemist in the Process Chemistry group at Merck for 4 years before going back to Wake Forest to become a physician associate. After graduating, she worked as a PA for over 10 years in Nephrology. Melissa and her husband currently work as entrepreneurs and live in Atlanta, GA with their three kids. Their most recent project is building and developing Cypress Micro Resort - a luxury resort immersed in nature- in the North Georgia mountains. |

|
Hengyu Li received his B.S. degree at Nankai University in 2016. Due to great interest on organic chemistry, he stayed to explore synthetic methodology through photoredox catalysis under Processor Yizhou Zhu for his M.S. degree. Since 2019, He joined WuXi AppTec and served as an associate senior scientist, dedicated to early-stage route development of API. |

|
Feng Peng joined the Process Research Department of Merck & Co., Inc. in 2012. His research at Merck focuses on using organic chemistry innovations to accelerate drug development and reduce the environmental footprint. He received his B. S. degree from Beijing Normal University. He obtained his M.S. under the supervision of Professor Dennis Hall at University of Alberta with a research focus on Boron Chemistry. Feng then moved to New York City, where he obtained Ph.D. in the area of total synthesis (Maoecrystal V) with Professor Samuel Danishefsky at Columbia University. |

|
Preston Mott received his B.S. degree in Chemistry from the University of California, at San Diego in 2019. He began his professional career working as a process chemist at Norac Pharma and then at Neurocrine Biosciences, working on early to late-stage route development of multiple API targets. He then enrolled at the California Institute of Technology for graduate school and joined the laboratory of Professor Sarah Reisman in 2023, focusing his research on natural product total synthesis. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved