Org. Synth. 2024, 101, 460-477
DOI: 10.15227/orgsyn.101.0460
Oxygen-Free Alkene Hydration Using Nitroarenes: Diastereoselective Hydration of Cholesteryl Acetate
Submitted by Jonas Elfert
1, Anup Bhunia
2, and Armido Studer*
1Checked by Ibrahim-Ethem Celik and Christopher D. Vanderwal
1. Procedure (Note 1)
A. Methyl 4-nitrobenzenesulfonate (1). A 500-mL single-neck round flask, equipped with a Teflon-coated stirring bar (3 cm x 0.5 cm), is charged with p-nosylchloride (22.2 g, 100 mmol, 1.0 equiv) (Note 2), which is dissolved in DCM (180 mL) (Note 3 and 4). A thermometer is fixed in the solution using a clamp. The solution is cooled to 0 ℃ using an ice bath and methanol (20 mL) (Note 5) is added (Figure 1A). Triethylamine (16.6 mL, 120 mmol, 1.2 equiv) (Note 6) is added dropwise over 40 minutes using a syringe pump (Note 7). The temperature did not exceed 10 ℃ during addition of the triethylamine to the reaction mixture. The thermometer is then removed and the reaction vessel is capped with a plastic stopper. The resultant mixture is stirred until no more starting material is observed (Figure 1B).
Figure 1. (A) Setup of the reaction before addition of triethylamine; (B) Setup with syringe pump; (C) Yellow solution after addition of all reagents (Photos provided by authors)
The reaction is monitored by thin layer chromatography (Note 8). After one more hour of stirring no more p-nosylchloride is observed by TLC (Note 9). Water (200 mL) is added and the mixture is transferred to a 500-mL separatory funnel (Figure 2A). The phases are separated and the aqueous phase is extracted with DCM (2 x 80 mL). The combined organic phases are washed with sat. NaCl solution (40 mL) and dried over MgSO4 (10 g) (Note 10) for 5 min. The drying agent is removed by filtration through a funnel fitted with a wool plug into a 500 mL flask and the filtration cake is washed with DCM (3 x 20 mL) (Figure 2B). The solvent is removed by rotary evaporator (40 ℃, 450 mm Hg), yielding the crude product as a yellow solid (18.5 g, 84 wt% pure) (Note 11).
Figure 2. (A) Separation of initial phases; (B) Filtration setup (Photos provided by authors)
The crude product is subjected to flash chromatography. A column (5 cm diameter) is wet-packed with silica (80 g) (Note 12) suspended in DCM to give a packed column with a height of 12 cm (Figure 3 A). The crude material is dissolved in DCM (50 mL) and loaded onto the column (Figure 3 B). The column is run with an overpressure of 75 mm Hg. After the dissolved product is fully absorbed onto the silica, the column is topped off with sand (2 cm) (Note 13) and DCM (300 mL) is added. The first 100 mL of eluted solvent are discarded and the following 200 mL are collected in a 250-mL flask (Figure 3 C) (Note 14).
Figure 3. (A) Packed column and crude product dissolved in DCM; (B) Crude product is loaded onto the column; (C) Column after 100 mL of DCM has eluted. Collection of the product is started at this point (Photos provided by authors)
The solvent is removed by rotary evaporator and the residue dried at high vacuum (0.08 mm Hg) overnight, yielding methyl 4‐nitrobenzenesulfonate as pale-yellow solid (16.2 g, 99 wt% purity, 73% yield) that melts at 90.7-91.6 ℃ (Figure 4) (Note 15).
Figure 4. Pale yellow 1 isolated after drying (Photo provided by authors)
B. 3β-Acetoxy-5α-cholestan-5-ol (2). An oven-dried 500-mL 3-neck flask (2 x NS 14/23, 1 x NS 29/32) is equipped with a Teflon-coated stirring bar (3 cm x 0.5 cm), a small and large septum and NS 14/23 glass-vacuum adapter (Figure 5 A). The flask is evacuated in vacuum until it reaches room temperature and then backfilled with argon (Note 16). The flask is charged with Fe(acac)3 (177 mg, 0.500 mmol, 0.025 equiv) (Note 17), cholesteryl acetate (8.57 g, 20.0 mmol, 1.0 equiv) (Note 18), methyl 4-nitrobenzenesulfonate (5.65 g, 26.0 mmol, 1.3 equiv) and NaHCO3 (3.36 g, 40.0 mmol, 2.0 equiv) (Note 19). The flask is evacuated and backfilled with argon once more time and dry methanol (80 mL) followed by dry THF (80 mL) (Note 20) (Note 21) are added (Figure 5 B). The red mixture is cooled to 0 ℃ with an ice bath and the small septum is exchanged for a quick-fit adapter fitted with a thermometer (Figure 5 C).
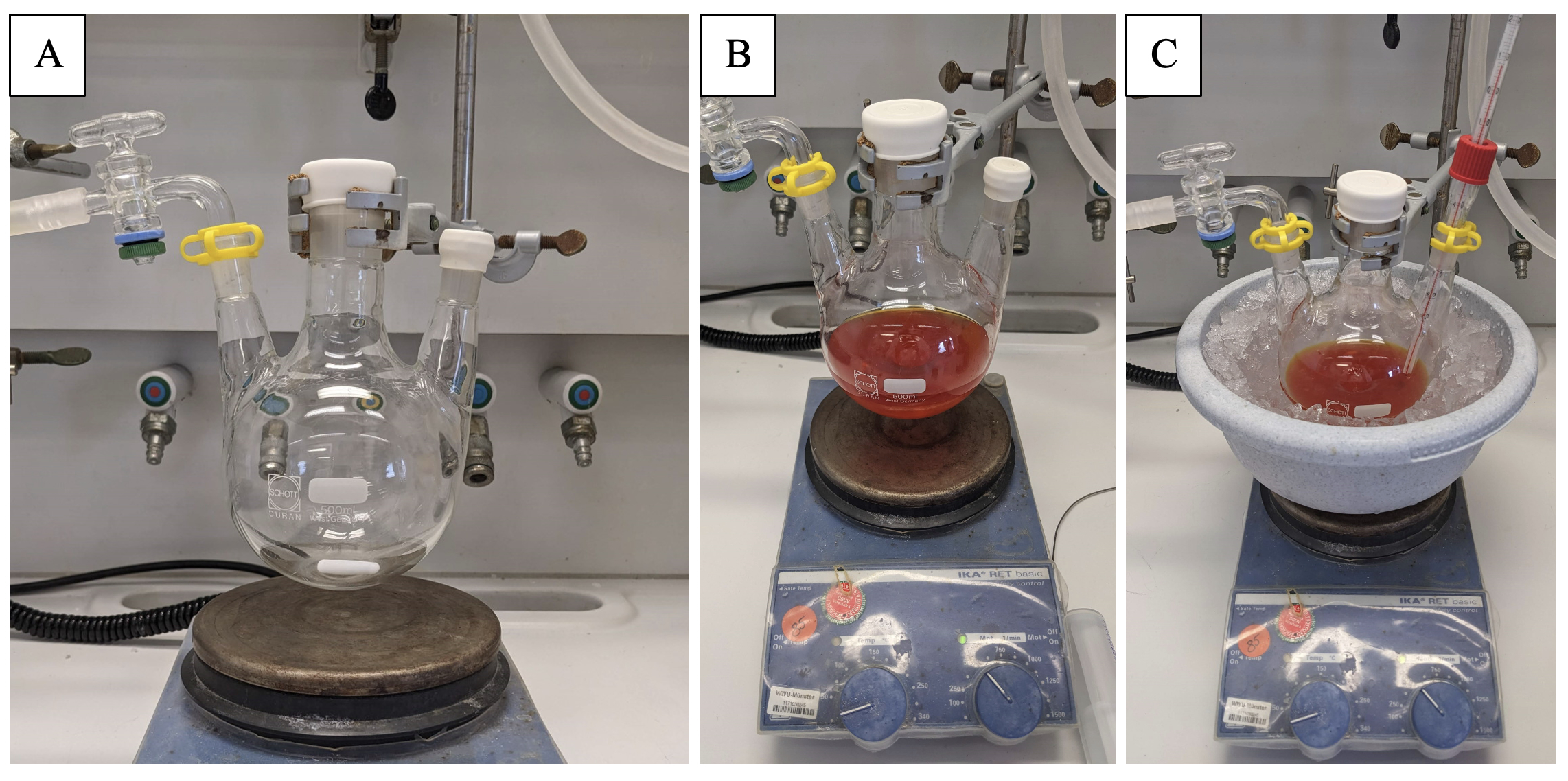
Figure 5. (A) Reaction setup; (B) Dissolved reactants prior to addition of phenylsilane; (C) Setup for cooling (Photos provided by authors)
Once the mixture is cooled down, phenylsilane (7.40 mL, 60.0 mmol, 3.0 equiv) (Note 22 and 23) is added dropwise over 12 minutes with a syringe, while the temperature does not exceed 10 ℃ (Note 24). After the addition is complete, the ice bath is removed, the quick fit is exchanged for a rubber septum again and the reaction is stirred overnight (16 h) (Figure 6A) (Note 25). Afterwards, the reaction flask is connected to the rotary evaporator and the solvents are removed (40 ℃, 185 mm Hg) (Figure 6 B).
Figure 6. (A) Reaction shortly after addition of phenylsilane and removal of the ice bath; (B) residue after removal of the solvent (Photos provided by authors)
To the black residue EtOAc (200 mL) (Note 26) and HCl (100 mL, 2 M) (Note 27) are added and the mixture is transferred to a 500-mL separatory funnel (Figure 7 A). The phases are separated and the aqueous phase is reextracted once with EtOAc (100 mL). The combined organic phases are washed with brine (100 mL) and then dried over MgSO4 (10 g) for 5 minutes. The drying agent is removed by filtration through a funnel fitted with a wool plug into a 500 mL flask and the filtration cake is washed with EtOAc (3 x 20 mL) (Figure 7 B). The solvent is removed by rotary evaporator (40 ℃, 110 mm Hg), giving a red slurry (Figure 7 C).
Figure 7. (A) Mixture transferred to a separatory funnel for extraction; (B) filtration setup; (C) Residue of workup after removal of solvents (Photos provided by authors)
The residue is purified by flash column chromatography. To this end, a column (5.5 cm diameter) is wet-packed with silica (150 g) suspended in eluent (pentane/EtOAc 90:10) (Note 28), giving a column packed to a height of 18 cm. The product is dissolved in DCM (150 mL) and 20 g of silica are added. The solvent is removed by rotary evaporator (40 ℃, 520 mm Hg) to obtain the dry load (Figure 8 A). This is loaded onto the column, wetted with eluent and topped of with sea sand (3 cm) (Figure 8 B). The column is charged with 1 L of eluent (pentane/EtOAc 90:10) and the fractions are collected in 100 mL tubes, filled to 80 mL (Note 29). The flash column is run with an overpressure of 0.1 bar. After the eluent passed, the column is charged again with 1 L of slightly more polar eluent (pentane/EtOAc 85:15). The fractions 13-20 are combined into a 1 L flask and the solvents removed by rotary evaporator (40 ℃, 520 mm Hg, then 110 mm Hg). The product is transferred to a 100-mL flask and after drying at high vacuum overnight, the product is obtained as white-pinkish solid (6.02 g, 94 wt% pure, 63% yield) (Figure 9) (Note 30).

Figure 8. (A) Preparation of column and crude material; (B) Loaded column (Photos provided by authors)
Figure 9. Product 2 after column chromatography (Photo provided by authors)
The remaining impurity can be removed by recrystallization. A 250 mL round flask is charged with a Teflon-coated stirring bar (2 cm x 0.5 cm) and the product (6.22 g). A reflux condenser is connected to the flask and ethanol (100 mL) (Note 31) is added and the mixture heated to reflux. Once all product has dissolved, water (10 mL) is added, leading to a suspension, and the mixture left to cool down. After cooling down, the flask is stored in a refrigerator overnight and then the mixture is filtrated through a Büchner funnel with a filtration paper (Figure 10 A). The filtration cake is washed with ice-cold ethanol (10 mL) and the solid is transferred to a 100-mL round flask. After drying overnight under high vacuum (0.075 mm Hg), the product is obtained as white, fine needles (5.02 g, 100 wt%, 56% yield) that melt at 185.9-186.6 ℃ (Figure 10 B) (Note 32 and 33).
Figure 10. (A) Filtration of recrystallized product; (B) Pure product 2 after drying (Photos provided by authors)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/about/governance/committees/chemical-safety.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
p-nosylchloride,
dichloromethane,
methanol,
triethylamine,
iron (III) acetylacetonate,
cholesteryl acetate,
sodium hydrogen carbonate,
tetrahydrofuran,
phenylsilane,
hydrogen chloride pentane,
ethyl acetate,
magnesium sulphate, silica gel and
chloroform.
2.
p-Nosylchloride (97%) was purchased from Sigma Aldrich and used as received.
3.
Dichloromethane (>99%) was purchased from Brenntag and freshly distilled before use.
4. Carrying out the reaction under argon and/or using ultra dry solvents does not affect the yield.
5.
Methanol (>99%) was purchased from Brenntag and distilled before use.
6.
Triethylamine (99%) was purchased from Acros Organics and used as received.
7. A syringe pump from KD Scientific was used, set to syringe diameter 20.1 mm, 0.415 mL/min, total time 40 min.
8. TLC plates were run using
pentane/
EtOAc 7:3 as eluent and visualization was done with a 254 nm UV lamp. Silica plates 60 F254 on glass-backing were purchased from Merck. R
f of
p-nosylchloride = 0.85, R
f of
1 = 0.59.
Figure 11. TLC Analysis after 1 h under the UV lamp (Photo provided by authors)
9. Running the reaction for a longer time decreases the yield. A repeated reaction with stirring for one hour longer dropped the yield by a total of 7%.
10.
Magnesium sulfate (dried) was purchased from Fisher Scientific and used as received.
11. Purity of the crude product was determined by QNMR
pdf, using
1,3,5-trimethoxybenzene as internal standard.
1,3,5-Trimethoxybenzene (>99.9%) was purchased from Sigma Aldrich and used as received. Second run by checkers: 17.2 g crude product, 84 wt% pure. Authors run: 17.0 g crude product (89 wt% pure).
12. Silica gel "Geduran Si 60" (0.040-0.063 mm) was purchased from Merck and used as received.
13. Sand was purchased from Honeywell and used as received.
14. The product front can be seen as a yellow band on the column. Collection of the product is started when the yellow band reaches the bottom of the column.
15.
Methyl 4-nitrobenzenesulfonate (
1) has the following properties: FTIR (neat): ν (cm
-1) 3108, 1607, 1535, 1455, 1404, 1353, 1186, 1111, 1094, 1011, 977, 861, 792, 747, 731, 683, 610.
1H NMR
pdf (400 MHz, CDCl
3) δ 8.43 - 8.39 (m, 2H), 8.13 - 8.09 (m, 2H), 3.85 (s, 3H).
13C NMR
pdf (100 MHz, CDCl
3) δ 151.0, 141.2, 129.5, 124.6, 57.1. HRMS (EI): calculated for C
7H
7NO
5S: 217.00394; found: 217.00398. m.p. 91-93 ℃. Purity of 99 wt% was determined by QNMR
pdf, using
1,3,5‐trimethoxybenzene as internal standard. The reagent could be stored in a flask away from sunlight at room temperature for several months without deteriorating. Second run by checkers: 15.1 g, 99 wt%, 69% yield. Authors run: 15.4 g, 70% yield, 99 wt%.
16. The whole reaction is carried out under protective gas atmosphere using a Schlenk line with argon overpressure, connected to a paraffin oil bubbler.
17. Fe(acac)
3 (>99%) was purchased from Thermo Scientific and used as received.
18.
Cholesteryl acetate (97%) was purchased from Acros Organics and used as received.
19.
Sodium hydrogencarbonate (≥99%) was purchased from Fisher Scientific and used as received.
20.
THF (≥99.9%, containing 250 ppm BHT as inhibitor) was purchased from Sigma Aldrich and was first refluxed over sodium and then distilled from potassium before use.
21. In the original procedure, only
methanol was used as a solvent. Here
THF is used as a cosolvent due to low solubility of the substrate.
THF is not necessary if the substrate is easily soluble in
methanol.
22.
Phenylsilane (97%) was purchased from Sigma Aldrich and used as received.
23. An excess of
phenylsilane is required to fully reduce the intermediate nitroso arene and prevent side product formation.
24. During addition, some hydrogen gas evolves.
25. To prevent build-up of pressure, the reaction is left connected to the Schlenk line with the argon turned off overnight. The Schlenk line is connected to a bubbler to release pressure and prevent air contamination.
26. Ethylacetate (>99.5%) was purchased from Brenntag and distilled before use.
27. HCl (37%) was purchased from VWR and diluted with distilled water before use.
28.
Pentane (>99%) was purchased from ExxonMobil and distilled before use.
29. TLC plates were run with
pentane/
EtOAc 9:1 as eluent. Visualization was done with a permanganate stain (1.5 g
KMnO4, 10 g K
2CO
3, 1.25 mL 10% NaOH, 200 mL water). R
f' of
2 = 0.35.
Figure 12. (A) TLC plate of crude product after visualization with KMnO4; (B) TLC plate of collected fractions 13-19 (Photos provided by authors)
30. Purity of
2 after column chromatography was by QNMR, using
1,3,5‐Trimethoxybenzene as internal standard. Checkers 2. run: 5.90 g, 97 wt% pure, 64% yield. Authors run: 6.22 g, 95 wt% pure, 66% yield.
31. Ethanol (99%, denaturized with 1% methyl vinyl ketone) was purchased from Walter-CMP and used as received.
32.
3β-Acetoxy-5α-cholestan-5-ol (
2) has the following properties: FTIR (neat): ν (cm
-1) 3446, 2935, 2867, 1735, 1704, 1468, 1380, 1364, 1254, 1028, 969, 906, 731, 649.
1H NMR
pdf (400 MHz, CDCl
3) δ 5.22 - 5.11 (m, 1H), 2.00 (s, 3H), 1.99 - 1.93 (m, 1H), 1.89 - 1.76 (m, 2H), 1.71 - 0.95 (m, 28H), 0.99 (s, 3H), 0.89 (d,
J = 6.6 Hz, 3H), 0.85 (dd,
J = 6.6, 1.8 Hz, 6H), 0.64 (s, 3H).
13C NMR
pdf (100 MHz, CDCl
3) δ 170.8, 75.1, 71.0, 56.4, 56.2, 45.7, 42.8, 40.2, 40.1, 39.6, 38.9, 36.3, 36.0, 34.9, 34.6, 30.7, 28.4, 28.1, 26.9, 26.0, 24.2, 24.0, 23.0, 22.7, 21.6, 21.4, 18.8, 16.2, 12.3. HRMS (ESI+): calculated for [C
29H
50O
3+Na]
+: 469.36522; found: 469.36521. m.p. 185-187 ℃. Purity of 100 wt% was determined by QNMR
pdf, using
1,3,5‐trimethoxybenzene as internal standard. The diastereoselectivity can be assigned by comparison with our previous publication.
833. Checkers second run: 4.90 g, 99 wt% pure, 54% yield. Authors first run: 5.24 g, 100 wt% pure, 59% yield. A second run on same scale resulted in 5.99 g of isolated compound in 95 wt% purity after column chromatography. Recrystallization yielded 4.95 g of product
2 pdf in 100 wt% purity.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The
Mukaiyama hydration is a well-established method in organic chemistry for the introduction of hydroxy groups into complex molecules.
3 Although the reaction is most commonly carried out with a cobalt catalyst and oxygen as oxidant, a plethora of methods has been published in the last decades, extending the reaction conditions with respect to precatalysts (Fe, Mn, Co), ligands (
β‐diketones, porphyrines, salen), reductants (silanes and borohydrides) and further parameters, although usage of oxygen as oxidant remained unchanged.
4,5 Notable alternative protocols are Boger's conditions where an iron phthalocyanine is used in combination with borohydrides as reductant and Shenvi's conditions where an optimized isopropoxy-substituted silane is used with improvements in yield and reaction rate.
6,7Compared to ionic hydration under acidic aqueous conditions, the radical Mukaiyama hydration profits from the significantly milder reaction conditions, which give rise to an excellent functional group tolerance and improved yields. The method does however suffer from the usage of molecular oxygen as oxidant, which can pose a problem for sensitive compound and is a possible hazard. Diastereoselectivities are also miniscule due to the oxygen being too small of a radical trap to show significant selectivity for less rigid substrates.
The method described here utilizes readily available nitroarenes as oxygen sources, circumventing the use of molecular oxygen entirely.
8 A metal hydride is formed in situ, which engages in an MHAT to the alkene. The thus generated carbon-centered radical is efficiently trapped by the nitroarene and further reduction leads to the oxygenated product. The method also has the upside of being highly diastereoselective for rigid systems, as is demonstrated with
cholesteryl acetate in this procedure, yielding only a single diastereoisomer of the hydration product. This renders this method highly valuable for late-stage functionalization and for the total synthesis of natural products.
Table 1. Selected examples from the scope. For the full scope see reference 8
This hydration protocol has already been used in a multitude of natural product syntheses, where it was shown to be a superior method for diastereoselective hydration.
9,10,11,12,13,14 In our publication we demonstrated that the hydration shows a high functional group tolerance and complete regioselectivity.
8 We could also show that compared to alternative hydration protocols superior yields and diastereoselectivities are generally achieved by using our method. A downside of this protocol is that the required methyl
p-nitrobenzenesulfonate is not commercially available, but it is easily prepared. Alternatively, cheaply available commercial
p-nosylchloride can also be used in the reaction, forming methyl
p-nitrobenzenesulfonate in situ. Unfortunately, not all substrates may support these slightly modified conditions.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
4-Nitrobenzenesulfonyl chloride; (98-74-8)
Triethylamine; (121-44-8)
Magnesium sulfate; (7487-88-9)
Iron(III) acetylacetonate; (14024-18-1)
Sodium hydrogencarbonate; (144-55-8)
Cholesteryl acetate; (604-35-3)
Phenylsilane; (694-53-1)

|
Jonas Elfert obtained his B.Sc. degree in chemistry from the University of Münster in 2018. He did a research stay at the group of Ruben Martin at the ICIQ, Spain. In 2020 he finished his master thesis in the group of Armido Studer, where he is currently pursuing his doctoral studies in organic chemistry. He is conducting research on radical reactions and novel hydrofunctionalizations. |

|
Anup Bhunia received his BSc in chemistry from Midnapore College, his MSc from the Indian Institute of Technology Guwahati, and his PhD from the CSIR-NCL under the guidance of Prof. Akkattu T. Biju. He then did postdoctoral studies as an Alexander von Humboldt fellow at the University of Münster in the lab of Prof. Armido Studer. In the summer of 2021, he started his independent career at the IISER Kolkata as a Ramanujan fellow. Subsequently, he joined IIT Hyderabad as an Assistant Professor in 2024. |

|
Armido Studer received his Diploma in 1991 and his Ph.D. in 1995 from ETH Zürich with Prof. Dieter Seebach. He then did postdoctoral studies at the University of Pittsburgh with Prof. Dennis P. Curran. In 1996 he started his independent career at the ETH Zürich. In 2000 he was appointed Associate Professor of Organic Chemistry at the Philipps-University in Marburg, and in 2004 Professor of Organic Chemistry at the University in Münster. |

|
Ibrahim-Ethem Celik received his BSc and MSc in chemistry from Goethe University in Frankfurt am Main. For his Ph.D. he joined in 2018 the group of Prof. Stefan F. Kirsch at Bergische Universität Wuppertal, where he worked on natural product synthesis of polyketides. In 2023, he joined the group Chris Vanderwal at University of California Irvine as a postdoctoral fellow, where he is currently working on total synthesis of a complex diterpenoid natural product. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved