Org. Synth. 2024, 101, 478-487
DOI: 10.15227/orgsyn.101.0478
Nitrosation of Diphenylamine with 3,3-Dimethyl-2-Nitroso-2,3-Dihydrobenzo[d]isothiazole 1,1-dioxide (NO-1)
Submitted by Pravien S. Rajaram, Keerthana Chakkanalil, and Ryan D. Baxter
1*
Checked by Haoran Xiong, Hirofumi Ueda, and Hidetoshi Tokuyama
1. Procedure (Note 1)
A. N-Nitrosodiphenylamine, (2). A 300 mL two-necked round bottom flask (Note 2) is used with one equipped with a thermometer to check the internal temperature and the other neck equipped with 29/32 condenser with 15/25 rubber septum on the top, which is pierced with an 18G vent needle (1.2×38 mm) and left open to the air. In the flask containing a 3.5 cm Teflon-coated magnetic stir bar, diphenylamine, 1 (1.50 g, 8.87 mmol, 1 equiv.) (Note 3) is dissolved in DCE (90 mL) (Note 4) (Figure 1A), followed by addition of 3,3-dimethyl-2-nitroso-2,3-dihydrobenzo[d]isothiazole 1,1-dioxide, NO-1, (2.60 g, 11.5 mmol, 1.3 equiv.) (Note 5) and the solution turns green (Figure 1B). The two-necked round bottom flask is placed in pre-heated oil bath at 90 ℃ and it takes 30 mins to reach the internal temperature to 85 ℃, the color of the reaction mixture changes from green to light brown (Figure 1C and 1D). Heating is continued for 20 hours and color of the reaction mixture turns dark orangish brown (Figure 2A).

Figure 1: A) Starting material dissolved in DCE; B) Color turns green on adding NO-1; C) Reaction set-up; D) Reaction mixture once the temperature reaches 85 ℃. (photos provided by checkers)
After 20 hours, the TLC is checked (Figure 3) (Note 6), the reaction is cooled to 25 ℃ (room temperature) and solvent is removed under reduced pressure (18 Torr). The flask containing the crude product is then charged with silica gel (10 g) and dichloromethane (20 mL) and the resultant suspension concentrated on a rotary evaporator (25 ℃ water bath, 18 Torr) (Note 7). The crude product, absorbed on silica gel, is added to a 550 mL chromatography column (7.0 cm ID X 20 cm tall) containing 300 g of silica gel wetted with 1:2 dichloromethane/hexane. The column is capped with 50 g of sand (Note 7) and eluted with 4000 mL 1:2 dichloromethane/hexane mixture (Rf = 0.2) as the mobile phase. The 150 mL fractions are collected in 200 mL Erlenmeyer flasks and the desired product is obtained in fractions 15-23 (Figure 2B) (Note 8). The isolated fractions are combined and concentrated under reduced pressure (18 Torr) at 25 ℃ to yield a yellow solid product, N-nitrosodiphenylamine 2 (1.52 g, 87% yield) (Figure 2C) with a melting point of 64 - 66 ℃. No special storage measures are required (Note 9) (Note 10) (Note 11).

Figure 2: A) Color of the reaction mixture after 20 h; B) TLCs from column chromatography; C) N-nitrosodiphenylamine isolated from the column (photos provided by checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/about/governance/committees/chemical-safety.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
diphenylamine,
1,2-dichloroethane and
N-nitrosodiphenylamine. Additional caution should be taken when using the highly toxic solvent
1,2-dichloroethane. The decomposition of the material releases nitric oxide, which can be harmful if inhaled. Extreme caution should be taken when working with all N-nitroso compounds, as some compounds in this class have been shown to be highly potent carcinogens.
22. All glassware were oven-dried before use.
3.
Diphenylamine (>99.0%) was purchased from Tokyo Chemical Industry Co., Ltd. and used as received.
4.
1,2-Dichloroethane (
DCE) (99.8%) was purchased from Aldrich Chemical Co., Inc. and used as received.
5.
NO-1 was synthesized from saccharin according to the procedure published from our lab.
3,4,56. On the TLC plate (Figure 3), the starting material (SM),
NO-1, co-spot (Co), and reaction mixture (RM) were compared with 1:1 DCM/hexane mixture. R
f value of the product equals 0.5 and was observed under the UV light.
Figure 3: Thin Layer Chromatography (TLC) analysis of the reaction mixture (RM) (photos provided by checkers)
7. Silica gel (Silica gel 60 N, 0.040-0.050 mm, spherical and neutral) was purchased from Kanto Chemical Co., Inc. and used as received.
8. Fractions containing
N-nitrosodiphenylamine 2 were identified using TLC analysis (Figure 2B). Using a solvent system of 1:2 dichloromethane/hexane,
N-nitrosodiphenylamine 2 has an R
f = 0.2 and can be visualized under UV light (254 nm).
9. Characterization data of the purified
N-nitrosodibenzylamine (
2):
1H NMR
pdf (600 MHz, CDCl
3) δ 7.52 - 7.50 (m, 2H), 7.47 - 7.44 (m, 1H), 7.42-7.41 (m, 4H), 7.35 - 7.31 (m, 1H), 7.10- 7.08 (m, 2H).
13C NMR
pdf (150 MHz, CDCl
3) δ 142.4, 136.6, 129.6, 129.4, 129.2, 127.2, 126.8, 119.5. IR (ATR) 3073, 3059, 1589, 1490, 1469, 1440, 1321, 1304, 1190, 1166, 1090, 1065 cm
-1. HRMS (ESI)
m/z = [M+H] calculated for C
12H
11N
2O
+ 199.0866, Found 199.0862.
10. The purity of product
2 was determined using
1H qNMR
pdf analysis.
1H qNMR
pdf was performed using a mixture of
2 (10.0 mg) and 1-bromo-3,5-dimethoxybenzene (10.0 mg) (Thermo Fisher Scientific Co., Inc., 97%, as an internal standard) in CDCl
3. The purity was calculated according to standard method as 97 wt%.
11. A second run on half-scale provided 750 mg (85%) of the product.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Nitric oxide (NO) is a small molecule of extreme biological importance. It has been implicated in a range of biological processes including vasodilation,
6 immune regulation,
7 neurotransmission,
8 and the inhibition of platelet aggregation.
9 Alkyl nitrites and nitrates are most often used as vasodilators, several
N-nitroso compounds are potent DNA alkylators that effectively halt tumor growth in certain cancers.
10Usual methods for nitrosation have involved the use of tert- butyl nitrite and inorganic nitrites, such as NaNO
2. This process requires strongly acidic conditions to generate electrophilic sources of NO.
11 These methods can be effective for the nitrosation of amides, secondary amines, and certain alcohols but the major drawback was rapid diazotization when reacting with primary amines.
12 In addition, NaNO
2 decomposes under basic conditions. In case of tert-butyl nitrite (TBN), an electrophilic trans-nitrosation reagent,
13 does not require strongly acidic conditions for trans-nitrosation, although some nucleophiles require excess TBN to minimize reversible trans-nitrosation with tert-butanol.
14 TBN has been effective for nitrosating amides, secondary amines, and certain alcohols but is known to oxidize primary alcohols under atmospheric conditions.
15,16Our lab has developed a new organic reagent that serves as an attractive alternative to TBN for the trans-nitrosation of nucleophiles under mild conditions. The
N-nitrososulfonamide reagent,
3,3-dimethyl-2-nitroso-2,3-dihydrobenzo[d]isothiazole 1,1-dioxide (
NO-1), is a an easily synthesized crystalline material.
4 Salient features of this method include readily available starting materials, broad functional group compatibility, good yields, and long-term integrity under ambient storage. Denitrosated
NO-1 precursor product 3,3-dimethyl-2,3-dihydrobenzo[d]isothiazole 1,1-dioxide (DMBS)
3 is easily recovered to regenerate
NO-1 with high fidelity after irreversible nitrosation reaction. Alkyl alcohols, amines, amides, ureas, and thiols are all effectively irreversibly nitrosated by
NO-1 under mild conditions, resulting in several nitroso compounds that are reported here for the first time (Table 1).
5 Hence, this protocol provides a modular access to
N-nitrosodiphenylamine through trans-nitrosation of commercially available nucleophile
diphenylamine utilizing
NO-1.
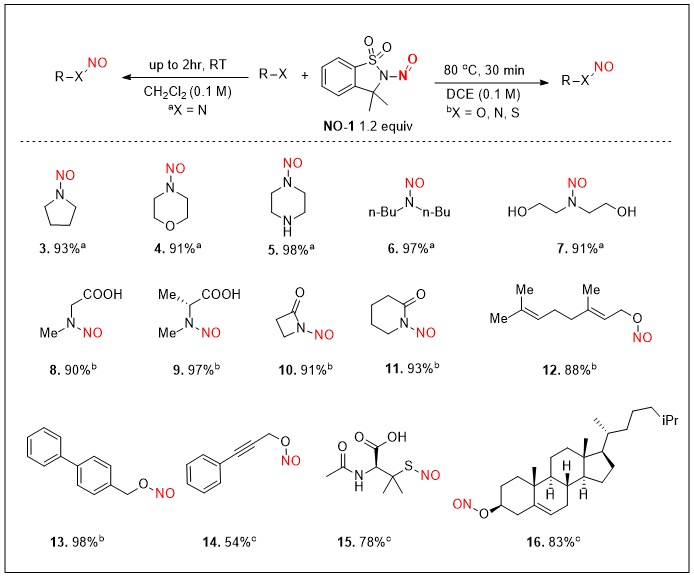
Table 1: General reaction conditions: a Substrate and NO-1 (1.2 equiv) in DCE (0.1 M) stirred at room temperature for 2 hours. b Substrate and NO-1 (1.2 equiv) in DCE (0.1 M) at 80 ℃ for 30 minutes. c Substrate and NO-1 (1.2 equiv) in ACN (0.1 M) at 80 ℃ for 30 minutes
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Diphenylamine; (122-39-4)
N-Nitrosodiphenylamine; (86-30-6)

|
Pravien is a 4th year PhD student. He came to the United States from Sri Lanka in 2016. He obtained his B.S. and M.S. from Fresno State, where he did research under the guidance of Dr. Qiao-Hong Chen. Currently under Dr. Ryan Baxter, Pravien works on the synthesis of cannabidiol analogs for vasodilator drug candidates, focusing on derivative synthesis of natural products and light-mediated reactions. |

|
Keerthana is a 2nd year PhD student. She holds an integrated master's degree from the National Institute of Science Education and Research (NISER), India. She currently works under Dr. Ryan Baxter, focusing on the synthesis of analogs of cannabidiolic acid and new organic chemistry methodology. |

|
Ryan D. Baxter received his B.S. in chemistry from the University of Wisconsin, Madison (2005) and an M.Sc. (2007) and Ph.D. (2010) from the University of Michigan. He performed postdoctoral research at The Scripps Research Institute (2011-2014). He is currently an Associate Professor and Chair of the Chemistry & Biochemistry department at the University of California, Merced. His research interests include single-electron transfers, synthetic methods, and luminescent organic materials. |

|
Haoran is a 2nd year PhD student. He was born in Yamaguchi, Japan in 1998. He obtained his B.S. (2021) and M.S. (2023) from the University of Tohoku (Pharmaceutical Sciences), where he did research under the guidance of Prof. Hidetoshi Tokuyama. His research interests include development of novel cyclization reactions and its application to total synthesis of complex natural products. |

|
Hirofumi Ueda received his Ph.D. (2010) from the Tohoku University under the direction of Professor Hidetoshi Tokuyama. After receiving the Ph.D., he started soon his academic carrier as an Assistant Professor in the same group. In 2018, he was promoted to lecturer and in 2023 to his current position of associate professor. He spent 7 months in 2019 at the University of California, Berkeley as a visiting scholar with Prof. Richmond Sarpong. His research interests center on the development of novel synthetic methodology involving oxidation, and applications to the synthesis of complex alkaloids and nitrogen-containing molecules. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved