Org. Synth. 2025, 102, 657-667
DOI: 10.15227/orgsyn.102.0657
Preparation of (S,S)-B2bg2, a Chiral Diboron Reagent
Submitted by Cliff Yang, Brooklyn Maddox, Anshu Yadav, Robert E. Maleczka Jr.*
1Checked by Samuel Jacobo and M. Kevin Brown
1. Procedure (Note 1)
(4S,4'S)-4,4'-Diethyl-2,2'-bi(1,3,2-dioxaborolane) ((S,S)-B2bg2) (1). A 100-mL round bottom flask and Teflon coated stir bar (egg-shaped 3/4 x 3/8'') were dried in an oven. The flask and stir bar were cooled to room temperature (22 ℃), charged with tetrahydroxydiboron B2(OH)4, (2.25 g, 25 mmol, 1.0 equiv) (Notes 1 and 2), and the flask was capped with a rubber septum. The flask was purged with N2 for 15 min (Figure 1A). Trimethyl orthoformate (11 mL, 10.6 g, 100 mmol, 4.0 equiv) (Note 3) was added via syringe (Figure 1B), and the reaction mixture was stirred briefly to form a suspension (Figure 1C). Acetyl chloride (40 μL, 39.3 mg, 0.50 mmol, 0.02 equiv) (Note 4) was added via syringe, and the reaction mixture was stirred until a clear solution was observed (typically 1-3 min, Figure 1D). (S)-1,2-Butanediol (4.5 mL, 4.5 g, 50 mmol, 2.0 equiv) (Note 5) was added via syringe, and the mixture was stirred for 30 min at room temperature (22 ℃).
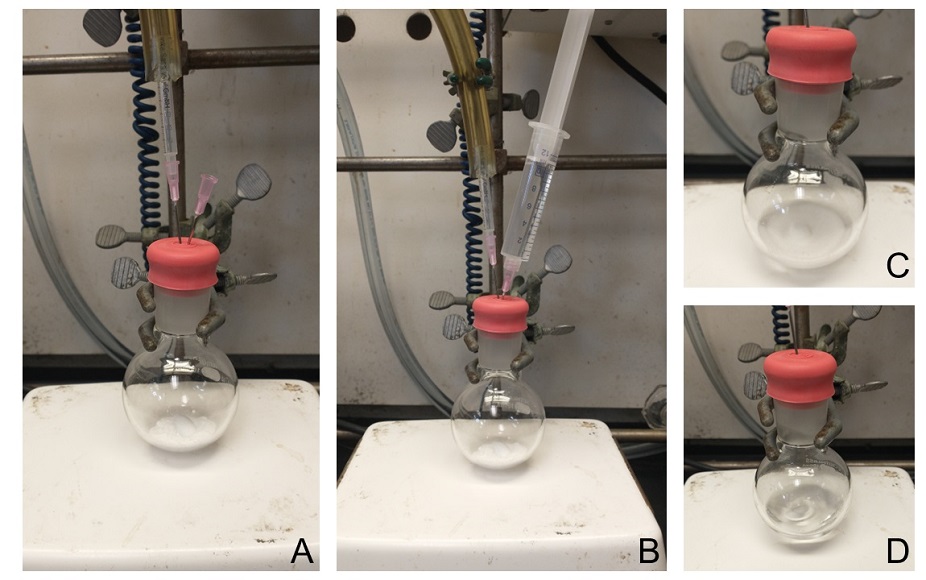
Figure 1. A. Purging of apparatus with N2; B. Injection of liquid compounds; C. Suspension formed before addition of acetyl chloride; D. Clear reaction mixture formed after addition of acetyl chloride
After 30 min, volatile solvents were removed on a rotary evaporator at 50 ℃ and 9 torr to yield a clear oil. The oil was further dried under high-vacuum (0.05 torr) for 18 h to remove additional volatile compounds.
Figure 2. Short path vacuum distillation apparatus
Further purification was conducted using short path distillation under vacuum. The crude product was transferred to a 50-mL, round-bottom flask equipped with a magnetic stir bar (egg-shaped 3/4 x 3/8''). A water-cooled short path condenser with a cow style distillation receiver was attached (Figure 2). The apparatus was evacuated under high vacuum (0.02 mmHg) and the flask containing the crude product was heated in an oil bath that was warmed to 175 ℃. A forerun at 92-96 ℃ (0.02 mm Hg) (approx. 0.5 mL) was collected and discarded before collecting the product at 92-96 ℃ (0.02 mm Hg). The product was collected as a clear oil (3.92 g, 79%) (Notes 6,7,8,9,10,11).
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regards to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/about/governance/committees/chemical-safety.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
acetyl chloride,
trimethyl orthoformate,
1,2-butanediol and the peroxide former
1,4-dioxane and
tetrahydroxydiboron, which has been reported to react with water to form
B(OH)3 and hydrogen gas (Wiberg, E.; Ruschmann, W, Über eine neue Borsäure ("Unnterborsäure") der Formel H
4B
20
4 und ihre Ester
Chem Ber. 1937,
6, 1393-1402; DOI: 10.1002/cber.19370700636)
.2.
Tetrahydroxydiboron was obtained from Ambeed and used as received.
3.
Trimethyl orthoformate (99.8%) was obtained from Sigma Aldrich and used as received.
4.
Acetyl chloride (98%) was obtained from Sigma Aldrich and used as received.
5.
(S)-1,2-Butanediol (97%) was obtained from Ambeed and used as received.
6. Characterization data for
1:
1H NMR
pdf (500 MHz, CDCl
3) δ 4.36 (p,
J = 7.0 Hz, 1H), 4.24 (ddd,
J = 9.4, 8.1, 1.4 Hz, 1H), 3.78 (ddd,
J = 8.9, 7.3, 1.4 Hz, 1H), 1.77 - 1.45 (m, 2H), 0.95 (td,
J = 7.5, 1.4 Hz, 3H).
13C NMR
pdf (126 MHz, CDCl
3) δ 78.6, 70.4, 29.0, 9.3.
11B NMR
pdf (160 MHz, CDCl
3) δ 30.8. HRMS (ESI): Calculated for C
6H
14O
4B
2Na [M-C
2H
3]
+: 195.0976, Found: 195.0971
7. The authors noted that the optical activity of
1 was measured to be -201.165° (DCM, 1 mg/mL, 22.1 ℃).
8. The authors noted that the purity of product
1 was determined using CHN analysis. Elemental composition of a sample of
1 was C (48.42%) and H (7.96%); expected from C
8H
16B
2O
4 is C (48.57%), H (8.15%).
9. The purity of product
1 was confirmed using
1H qNMR
pdf analysis.
1H qNMR
pdf was performed using a mixture of
1 (24.0 mg) and hexamethyl benzene (22.1 mg) (Sigma Aldrich, 99%, as an internal standard) in CDCl
3. The purity was calculated according to the standard method as 98.3 wt%.
10. The authors noted that the enantiomeric purity of the product could not be determined via the use of chiral NMR shift reagents, chiral GC, or chiral supercritical fluid chromatography (SFC). All analyses of a mixture of
B2Bg2 prepared with racemic 1,2-butanediol did not separate the three stereoisomers expected. While the reaction is very unlikely to result in epimerization of the stereocenters present in the enantiomerically pure alcohol, an indirect method of establishing the enantiomeric purity of the diboron product was required (see
note 11).
11. Enantiomeric purity of
1 was assessed by the checker by analyzing the products of Miyaura borylations with B
2bg
2 and
4-bromoaniline to form
2 and
3. The detailed procedure is in
Note 12. HPLC analysis of the product prepared using
1 resulted in one product peak (
2). HPLC analysis (Notes 13) of the product prepared using the mixture of B
2bg
2 stereoisomers resulted in two peaks corresponding with the two enantiomers produced
2 and
3.
12. Procedure for Miyaura borylation (Scheme 1): An oven-dried 100 mL round bottom flask was charged with
4-bromoaniline (172.0 mg, 1 mmol, 1 equiv) (
Note 15), B
2bg
2 (237.4 mg, 1.2 mmol, 1.2 equiv),
Pd(dppf)Cl2•CH2Cl2 (0.82 g, 0.1 mmol, 0.1 equiv) (
Note 16), and
KOAc (294.4 mg, 3 mmol, 3 equiv).
1,4-Dioxane (8.3 mL) and a stir bar were added, and the round bottom flask was fitted with a water-cooled condenser. The condenser was fitted with a septum and the apparatus purged with
N2 for 15 min. The reaction was heated at 90 ℃ for 12 h under
N2. After cooling, the mixture was filtered through Celite, and volatile solvents were evaporated. The product was purified via silica gel chromatography with 20/80
ethyl acetate/
hexane with 1%
triethylamine added (
Note 14). This procedure is based on previously reported literature.
2Scheme 1. Miyaura borylation of 4-bromoaniline with B2Bg2
13. Miyaura borylation of
4-bromoaniline with
1 resulted in
2 (0.120 g, 63%). HPLC analysis (ChiralPak IA with an amylose tris(3,5-dimethylphenylcarbamate) immobilized 5 μm column) showed one peak in the chromatogram (Figure 3A). Miyaura borylation of
4-bromoaniline with a mixture of B
2bg
2 prepared from racemic 1,2-butanediol resulted in a mixture of
2 and
3 (0.111 g, 58%). HPLC analysis (ChiralPak IA with an amylose tris(3,5-dimethylphenylcarbamate) immobilized 5 μm column) showed two peaks in the chromatogram (Figure 3B).
Figure 3. Chiral HPLC chromatograms; A. Racemic mixture 2 and 3. B. Compound 2 from procedure.
14. Characterization data for
2:
1H NMR
pdf (500 MHz, CDCl
3) δ 7.63 (d,
J = 8.5 Hz, 2H), 6.66 (d,
J = 8.4 Hz, 2H), 4.47 (dq,
J = 7.9, 6.6 Hz, 1H), 4.37 (dd,
J = 8.9, 7.6 Hz, 1H), 3.92 (dd,
J = 8.8, 6.8 Hz, 2H), 1.80 - 1.58 (m, 2H), 1.00 (t,
J = 7.4 Hz, 3H).
13C NMR
pdf (126 MHz, CDCl
3) δ 149.5, 136.6, 114.2, 78.4, 70.7, 28.9, 9.0.
11B NMR
pdf (160 MHz, CDCl
3) δ 31.2.
15.
4-Bromoaniline (99%) was obtained from Oakwood Chemical and used as received.
16.
Pd(dppf)Cl2•CH2Cl2 (99%) was obtained from Strem and used as received.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Diboron reagents are widely used as boron sources in borylation reactions (Figure 4). Due to the stable products produced, B
2pin
2 is often chosen. Other smaller boron sources such as B
2eg
2 have been found to improve selectivity in ortho directed Ir-catalyzed CH borylation of anilines and phenols, though transesterification of the Beg to the Bpin group post-borylation is required for isolation of a stable product.
3,4 Another boron source, B
2bg
2, provides a balance, affording high ortho selectivity without the need for post-borylation transesterification.
5 These boron sources can all be prepared from
tetrahydroxydiboron via a simple, green reaction.
6Figure 4. Common diboron sources for borylation
Previous preparations of B
2bg
2 and B
2pg
2 used racemic 1,2-butanediol, resulting in a mixture of 3 stereoisomers. These stereoisomers (
R,
S), (
R,
R), and (
S,
S) are indistinguishable by NMR, though the stereoisomers of B
2pg
2 could be observed by NMR in the presence of chiral shift reagents.
6 Using enantiomerically pure (
S)-1,2-butanediol, (
S,
S)-B
2bg
2 (
1) can be produced. While the synthesis of the single stereoisomer of B
2bg
2 is as simple as the synthesis of multiple stereoisomers, establishing the stereoisomeric purity is difficult.
In contrast with B
2pg
2, the stereoisomers of B
2bg
2 could not be distinguished by NMR even in the presence of chiral shift reagents or separated via GC or SFC chromatography using various chiral columns. However, when a mixture of three stereoisomers of B
2bg
2 were used in a Miyaura borylation of 4-bromoaniline following a known procedure,
2 the resulting enantiomers
2 and
3 could be separated via HPLC chromatography. When
1 was used in the same borylation, only
2 was observed. This demonstrates the utility of diboron reagents beyond iridium catalyzed borylation and the stereoisomeric purity of the (
S,
S)-B
2bg
2 produced as described in this report.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Tetrahydroxydiboron (13675-18-8)
Trimethyl orthoformate (149-73-5)
Acetyl chloride (75-36-5)
(S)-1,2-Butanediol (73522-17-5)
4-Bromoaniline (106-40-1)
Pd(dppf)Cl2•CH2Cl2; [1,1-Bis(diphenylphosphino)ferrocene] dichloropalladium(II), dichloromethane complex (95464-05-4)
KOAc; Potassium Acetate (127-08-2)
1,4-Dioxane (123-91-1)
Ethyl acetate (141-78-6)
Hexane (110-54-3)
Triethylamine (121-44-8)

|
Cliff Yang earned his B.S. in chemistry from Hillsdale College in 2022. While at Hillsdale, he participated in an NSF REU program at Michigan State University. That experience helped to guide his graduate school plans, as he is currently a third year Ph.D. student in organic chemistry at Michigan State University where he works on iridium-catalyzed borylations with Prof. Maleczka. |

|
Brooklyn Maddox is a junior at Michigan State, majoring in chemistry with a math minor. She is an undergraduate researcher in the Maleczka group and a goalie on MSU's women's hockey team. |

|
Anshu Yadav did his BSc (Hons.) in chemistry from University of Delhi. Later he pursued MS from Indian Institute of Technology, Bombay where he worked on radical mediated cascade cyclisation of vinylogous carbonate to tetra-substituted pyran in the lab of Dr. Santosh Gharpure. After completing his MS degree, he moved to Michigan State University to pursue a Ph.D. in chemistry in the labs of Profs. Robert E. Maleczka Jr. and Milton R. Smith III. His main research focus is on the development of new methodology for iridium catalyzed C(sp3)-H borylations. |

|
Robert E. Maleczka, Jr. is a Professor of Chemistry at Michigan State University (MSU). He received a B.S. in chemistry from the University of Illinois, and then spent three years in the anti-infective discovery group at Abbott Laboratories, before undertaking graduate studies at the Ohio State University. After receiving his Ph.D., he moved to the University of Pennsylvania as an American Cancer Society post-doctoral fellow. In 1995, he began his independent career at MSU. The Maleczka group's research interests include the invention of "green" reactions and strategies for organic synthesis, with a particular focus on boron and silicon containing compounds. |

|
Samuel Jacobo earned his B.S. in Chemistry at Stanford University where he worked under Dr. Malhotra at the Stanford School of Medicine. There he helped synthesize chemotherapy derivatives that helped mediate radiation toxicity. He then completed his M.S. at the University of San Francisco where he worked under Dr. Herman Nikolayevskiy. There he helped develop a novel method to selective DNA-alkylation of cancerous cells. Following these experiences, he is now a 3rd year graduate student at Indiana University - Bloomington. He currently works under Dr. M. Kevin Brown in the field of Ni-catalyzed carboboration of unactivated alkenes. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved