Org. Synth. 2003, 80, 177
DOI: 10.15227/orgsyn.080.0177
PREPARATION OF α-ACETOXY ETHERS BY THE REDUCTIVE ACETYLATION OF ESTERS: endo-1-BORNYLOXYETHYL ACETATE
[(Ethanol, 1-[[(1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-yl]oxy]-, acetate)]
Submitted by David J. Kopecky and Scott D. Rychnovsky
1.
Checked by Guillaume L. N. Péron, Cédric R. N. Fischmeister, and Andrew B. Holmes.
1. Procedure
A flame-dried, 2-L, three-necked, round-bottomed flask, equipped with a large Teflon magnetic stirring bar and three rubber septa, is charged with 5.5 g (28.1 mmol) of (−)-bornyl acetate (Note 1) in 162 mL of dichloromethane (Note 2) under an argon atmosphere. A thermocouple (Note 3) is immersed in the solution through one of the rubber septa. The flask is placed in a dry ice/acetone bath and cooled to an internal solution temperature of −78°C. Stirring is begun and diisobutylaluminum hydride, 54.4 mL of a 1.0M solution in hexanes (54.4 mmol, 2 eq), is added as a slow stream via a glass syringe over a period of 13 min; during this addition, the internal solution temperature does not exceed −72°C (Notes 1,4). The clear solution is stirred for an additional 45 min at −78°C, then 6.6 mL of pyridine (6.5 g, 81.6 mmol, 3.0 eq) is added dropwise via a glass syringe, maintaining the internal temperature below −76°C. Immediately following, a solution of 6.5 g of 4-dimethylaminopyridine (DMAP) (54.5 mmol, 2.0 eq) in 80 mL of dichloromethane (Note 2) is added slowly via an 18-gauge stainless steel cannula (Note 5). The internal temperature is not allowed to rise above −72°C during this addition. Acetic anhydride (15.4 mL, 16.6 g, 163.2 mmol, 6.0 eq) is then added dropwise via a glass syringe over 5.5 min at a rate to maintain the internal temperature below −72°C.
The resultant light yellow solution is stirred at −78°C for 12 hr (Note 6). During this time, the color of the solution intensifies considerably. The bright yellow solution is warmed to 0°C in an ice water bath and stirred for 35 min, during which time the color of the solution becomes bright yellow-orange. The reaction is then quenched by the sequential addition of 270 mL of saturated aqueous ammonium chloride and 200 mL of saturated aqueous sodium potassium tartrate. The emulsion is removed from the ice water bath and stirred vigorously for 50 min, by which time adequate phase separation is observed (Note 7). The biphasic mixture is transferred to a 2-L separatory funnel and is extracted with four 175-mL portions of dichloromethane. The combined extracts are washed sequentially with two 500-mL portions of an ice-cooled 1M aqueous solution of sodium bisulfate, three 500-mL portions of saturated aqueous sodium bicarbonate and 500 mL of saturated aqueous sodium chloride. The solution is dried over anhydrous sodium sulfate, filtered, and is concentrated under reduced pressure to afford 8.5 g of a yellow oil.
The residue is purified using flash chromatography with 145 g of triethylamine-deactivated Merck 9385 silica gel 60 (230-400) (Note 8) eluting with 5% ethyl ether in hexanes (Note 9). Fractions of 50-mL are collected, and product-containing fractions are identified by TLC analysis (Note 10). The product (6.4 g, 95%) is obtained as a clear oil (Notes 11,12). 1H NMR and 13C NMR spectra indicate that the product is a 2.8:1 mixture of diastereomers. Short path vacuum distillation of a portion of the product afforded material of comparable purity, bp 73.5−75°C (2 mm).
2. Notes
1.
(−)-Bornyl acetate, purchased from Aldrich Chemical Company, Inc., was of 97% purity and was used as received.
Diisobutylaluminum hydride (DIBALH) (1.0M solution in hexanes) and 4-dimethylaminopyridine were purchased from Aldrich Chemical Company, Inc. and used as received.
Pyridine was purchased from Aldrich Chemical Company, Inc. and was distilled from calcium hydride at atmospheric pressure prior to use.
Acetic anhydride was purchased from Fisher Scientific, Inc. and was distilled at atmospheric pressure prior to use.
2.
The checkers obtained dry
dichloromethane by distillation from
calcium hydride. The submitters dried
dichloromethane through an alumina filtration system
2.
3.
A
Digi-Sense digital thermometer was used. The internal temperature needs to be monitored, and the additions should be slow enough to avoid significant exotherms. The checkers found that temperature control is the major factor influencing the outcome of the reaction. The initial procedure was submitted as double scale [
i.e.
11 g (56.2 mmol) (−)-bornyl acetate]. However, the checkers found temperature control on full scale to be difficult, resulting in incomplete reaction and/or over-reduction of the
bornyl acetate. The checkers found that the procedure is reliably reproducible on half scale (
5.5 g (−)-bornyl acetate).
4.
The checkers found that reproducible results were obtained when a freshly opened bottle of
diisobutylaluminium hydride was used.
5.
The cannula transfer was accomplished by briefly evacuating the reaction flask using a vacuum line connected to a
vacuum pump, and then pressurizing the flask containing the DMAP solution with just over 1 atmosphere of
argon.
6.
A bath temperature of −78°C was maintained during the 12 hr period by packing the bath with dry ice and covering the bath and reaction flask with aluminum foil.
7.
Although the two phases separated, the aqueous phase remained cloudy.
8.
The submitters used ICN 230-400 mesh silica gel. The silica gel was deactivated by mixing it with
325 mL of 2% (v/v) triethylamine in hexanes. The resultant slurry was poured onto the column, packed, and then flushed with
200 mL of 5% ethyl ether in hexanes to remove residual triethylamine before product loading.
9.
The head pressure of the flash column during chromatography was 2.5 psi.
10.
The α-acetoxy ether elutes with an R
f of 0.32 on Merck silica gel F
254 in
5% (v/v) ethyl ether in hexanes (product stains yellow-green in an acidic solution of
p-anisaldehyde).
11.
The submitters obtained the equivalent of
6.4 g (
95%). Physical and spectroscopic properties are as follows: IR (mixture of isomers, neat) cm
−1: 2952, 2879, 1737, 1452, 1372, 1247, 1175, 1141, 1072, 1033, 1009, 929, 833;
1H NMR (500 MHz, CDCl
3, mixture of isomers) δ: 0.81-0.85 (m, 9 H), 0.99 (dd, 1 H, J = 13.1, 3.4, minor isomer), 1.10 (dd, 1 H, J = 13.3, 3.4, major isomer), 1.16-1.28 (m, 2 H), 1.38 (d, 3 H, J = 5.4, minor), 1.40 (d, 3 H, J = 5.3, major), 1.59 (t, 1 H, J = 4.5, major). 1.64 (t, 1 H, J = 4.5, minor), 1.63-1.74 (m, 1 H), 1.90-2.01 (m, 1 H), 2.04 (s, 3 H, major), 2.05 (s, 3 H, minor), 2.07-2.19 (m, 1 H), 3.81 (ddd, 1 H, J = 9.9, 3.2, 2.0), 5.93 (q, 1 H, J = 5.2, minor), 5.94 (q, 1 H, J = 5.2, major);
13C NMR (125 MHz, CDCl
3, mixture of isomers) δ: 13.4 (minor isomer), 13.6 (major isomer), 18.8 (major), 18.8 (minor), 19.7 (major), 19.7 (minor), 20.9 (major), 21.2 (minor), 21.4 (minor), 21.6 (major), 26.4 (minor), 26.6 (major), 28.1 (major), 28.2 (minor), 36.1 (minor), 37.0 (major), 44.9 (minor), 45.0 (major), 47.2 (major), 47.6 (minor), 48.8 (minor), 49.3 (major), 82.6 (minor), 85.9 (major), 95.3 (minor), 97.7 (major), 170.6 (major), 170.9 (minor). Anal. Calcd for C
14H
24O
3: C, 70.0; H, 10.1. Found: C, 70.0; H, 10.0. The submitters obtained C, 70.0; H, 10.0.
12.
The product contained a 3% impurity of
(−)-bornyl acetate as indicated by the presence of the following diagnostic peaks in the
1H NMR spectrum: 0.87 (s, 3 H), 0.90 (s, 3 H), 0.96 (buried dd, 1 H), 2.06 (s, 3 H). This impurity originates from incomplete reduction and/or over-reduction.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The reductive acetylation of esters and lactones can be accomplished by treatment of the ester with a two-fold excess of DIBALH at −78°C followed by trapping of the resulting aluminum hemiacetal intermediate with
acetic anhydride in the presence of
pyridine and DMAP at low temperature.
3 This transformation has been successfully applied to a wide range of esters and lactones, including sterically-hindered acyclic esters, β-alkoxy esters, benzyl esters and macrolactones. A compilation of representative examples is presented in Table I.
3a Upon exposure to mild acid, the α-acetoxy ether products undergo facile conversion to the corresponding oxacarbenium ions, which can be trapped by a wide variety of carbon- or heteroatom-based nucleophiles.
3b For example,
(endo)-1-bornyloxyethyl acetate can be efficiently allylated by treatment with
boron trifluoride etherate and
allyltrimethylsilane (82% yield; d.r. = 3.7:1), or ethylated with
trimethylsilyl triflate and
diethylzinc (87% yield; d.r. = 1.9:1).
4 α-Acetoxy ethers have also been utilized as Prins cyclization precursors in our studies toward the diastereoselective synthesis of polyfunctionalized tetrahydropyran ring systems.
5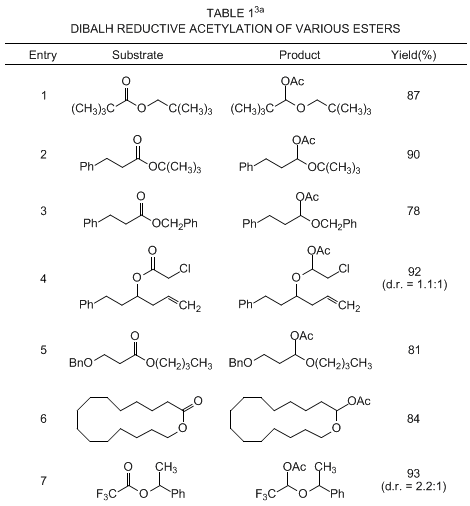
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
endo-1-Bornoxyethyl acetate:
Ethanol, 1-[[(1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]-hept- 2-yl]oxy]-, acetate (9); (284036-61-9)
(−)-Bornyl acetate:
Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S,2R,4S)- (9); (5655-61-8)
Diisobutylaluminium hydride:
Aluminum, hydrobis(2-methylpropyl)- (9); (1191-15-7)
Pyridine (8, 9); (110-86-1)
4-(Dimethylamino)pyridine:
4-Pyridinamine, N,N-dimethyl- (9); (1122-58-3)
Acetic anhydride:
Acetic acid, anhydride (9); (108-24-7)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved