Org. Synth. 2005, 82, 59
DOI: 10.15227/orgsyn.082.0059
1-(TERT-BUTYLIMINO-METHYL)-1,3-DIMETHYL-UREA HYDROCHLORIDE
[Urea, N-[[(1,1-dimethylethyl)imino]methyl]-N,N'-dimethyl-, Monohydrochloride]
Submitted by David D. Díaz
1, Amy S. Ripka
1, and M. G. Finn
1.
Checked by Daniel Laurich, Günter Seidel, and Alois Fürstner.
1. Procedure
Caution! Use safety glasses and nitrile gloves under a well-ventilated hood since isocyanides have pungent odors and some are known to be toxic. A 1-L, three-necked, round-bottomed flask is equipped with a mechanical stirrer (Note 1), a vacuum take-off adapter attached to a nitrogen source, and a pressure equalizing dropping funnel closed with a glass stopper. The flask is flame-dried, cooled under nitrogen, and charged with tetrahydrofuran (THF) (250 mL) (Note 2) and tert-butyl isocyanide (12.4 mL, 110 mmol) (Note 3). To this homogeneous solution is added acetyl chloride (8.6 mL, 121 mmol) (Note 4) and the mixture is vigorously stirred for 15 min. A solution of 1,3-dimethylurea (24.2 g, 275 mmol) (Note 3) in 150 mL of THF is then introduced slowly and continuously through the dropping funnel. After 15-20 min, a white precipitate appears (Note 5). The reaction mixture is stirred for 14 h at room temperature, after which time the heterogeneous mixture is filtered through a Buchner funnel connected to vacuum. The white precipitate is washed with cold THF (Note 6) to remove excess urea, the acetylurea byproduct and colored impurities (Note 7). The product is dried in a vacuum oven at 50°C for 20 h to give 19-20 g (85-90%) of analytically pure product as a white powder (Notes 8-10). The hygroscopic product should be stored under nitrogen.
2. Notes
1.
The submitters used a
magnetic stir bar (50 mm in length × 7 mm in diameter) instead of the
mechanical stirrer but noted that stirring may become difficult as the reaction proceeds. This problem is avoided when a
mechanical stirrer is used.
2.
The submitters used commercial
tetrahydrofuran, grade OPTIMA (Fisher). The checkers used THF dried by distillation over Mg-
anthracene under
argon.
3.
tert-Butyl isocyanide (≥98%, Fluka) and
1,3-dimethylurea, 98% (Acros) were used as received.
4.
Acetyl chloride, p.a., was used as supplied by Acros, and dispensed by syringe. For the scale proposed it is not necessary to distill this reagent, although this is recommended for smaller scale reactions.
5.
The submitters noted that stirring becomes difficult shortly after the precipitation starts if a
magnetic stir bar is used.
6.
THF at 0°C is necessary for the washing process as follows: suction is stopped and the white solid is suspended completely in cold
THF, the suction is turned on to remove the liquid, and the operation is repeated several times until 1 L of cold
THF is used. After washing, air is pulled through the solid for 20 min to dry the product, and clumps are broken manually with a
spatula. The solid remaining on the walls of the funnel is dissolved in
CH2Cl2 and the solvent evaporated under vacuum. The two fractions of solid are combined.
7.
The byproduct
N-acylurea may be isolated in equimolar amounts to
formamidine urea by column chromatography on
silica gel (
7:3 EtOAc:hexanes, R
f ca. 0.5) of the residue from the combined filtrates.
8.
The checkers dried the product
in vacuo (10
−3 Torr) at ambient temperature for 3 h.
9.
Spectral and analytical data:
mp 172-173°C;
1H NMR
pdf (400 MHz, CDCl
3): δ 11.09 (d,
J = 14.8 Hz, 1 H), [8.72 (d,
J = 15.2 Hz), 8.7 (m); 2 H], 3.85 (s, 3 H), 2.94 (d,
J = 4.4 Hz, 3 H), 1.59 (s, 9 H);
13C NMR (100 MHz, CDCl
3): δ 152.8, 150.7, 58.7, 34.7, 29.1, 28.0; IR (film): 3209, 2984, 1722, 1671, 1535 cm
−1. Anal. calcd for C
8H
18ClN
3O: C, 46.26; H, 8.74; Cl, 17.09; N, 20.23. Found: C, 46.14; H, 8.63; Cl, 17.10; N, 20.29.
10.
As a precaution, the formamidine urea should be handled with care in a well-ventilated hood.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Formamidines are of interest in synthetic chemistry
2 and have been used extensively as pesticides. The present procedure is an example of a novel process in which the addition of a substituted urea to a mixture of isocyanide and acid chloride gives formamidine urea salts
1 in pure form.
3 The yields of
1 are maximized by the use of
2.5-3.0 equiv of urea, and
THF provides the best results in general. The process was found to be tolerant of substantial variations in the nature of the components, but restricted almost exclusively to acid chlorides. A proposed mechanism for the formation of
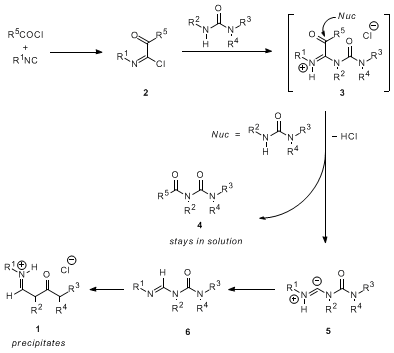
1 is shown in Scheme 1.
3 The first key intermediate is the electrophilic adduct of isonitrile and acid chloride,
2. Substitution of chloride by urea at the chloroiminium carbon gives
3. The unique aspect of the mechanism is then proposed to be capture of
3 at the active carbonyl carbon by another equivalent of
urea to form the soluble
acylurea 4 and intermediate
5, which may be formulated as the betaine shown or a stabilized carbenoid. Structure
5 should undergo rapid proton transfer to give the
formamidine 6, and an equivalent of
HCl is extracted by precipitation of the hydrochloride salt
1. The efficiency and convenience of the reaction is governed by both the generation of the reactive adducts and the precipitation of the final product. The overall transformation is made possible by the ability of the acid chloride to change the nature of the isonitrile carbon from nucleophilic to electrophilic. The entry to the formamidine urea skeleton exemplified here is quite a bit more convenient than previous methods.
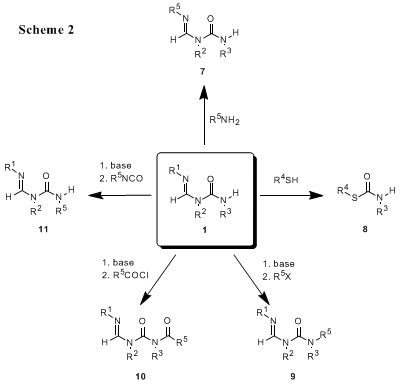
The
tert-butylamino fragment of
1 may be readily exchanged with a wide variety of primary nitrogen nucleophiles,
4 allowing the easy preparation of a variety of formamidine ureas (
7, Scheme 2). Formamidine ureas also undergo substitution at the carbonyl carbon to give thiolcarbamates (
8) and the formamidine urea nucleus can be elaborated by base-mediated alkylation and acylation (
9,
10,
11).
5
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
1,3-Dimethylurea:
Urea, N,N'-dimethyl-; (96-31-1)
Acetyl chloride; (75-36-5)
tert-Butyl isocyanide:
Propane, 2-isocyano-2-methyl-; (7188-38-7)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved