Org. Synth. 2006, 83, 49
DOI: 10.15227/orgsyn.083.0049
FRAGMENTATION-RECOMBINATION NAZAROV CYCLIZATION: 3,4-DIMETHYLCYCLOPENT-2-EN-1-ONE
Submitted by Keith D. Schwartz and James D. White
1.
Checked by Shin-ya Tosaki and Masakatsu Shibasaki.
1. Procedure
A. Isopropyl (E)-but-2-enoate (1) A 500-mL round-bottomed flask is fitted with a Soxhlet extractor containing magnesium sulfate (10 g) in a cellulose extraction thimble. Atop the extractor is a reflux condenser fitted with a rubber septum and an argon line. The flask is charged with crotonic acid (17.00 g, 197.5 mmol), isopropanol (106 mL, 1.39 mol), concentrated sulfuric acid (2.5 mL) and benzene (30 mL) (Note 1). The mixture is heated to reflux over the MgSO4 plug for 18 h and is then cooled to room temperature. Benzene (60 mL) is added, followed by the dropwise addition of a 10% aqueous solution of sodium bicarbonate (100 mL) while stirring. The mixture is transferred to a separatory funnel, the layers are separated, and the aqueous portion is extracted with benzene (3 × 25 mL). The organic layers are combined, washed with brine (50 mL), dried over anhydrous sodium sulfate, and filtered. The solvent is evaporated under vacuum and the resulting yellow oil is purified by distillation (46–48 °C/25 mmHg) to give 17.98–18.00 g (71%) of isopropyl (E)-but-2-enoate (1) (Note 2) as a colorless oil.
B. 3,4-Dimethylcyclopent-2-enone (2) A three-necked, 1-L round-bottomed flask is fitted with a mechanical stirrer, a glass stopper, a rubber septum, and an argon line. The flask is charged with phosphoric acid (32.0 g) and phosphorus pentoxide (48.0 g) (Note 1). The mixture is stirred at 100 °C for 1 h, at which time it becomes homogenous. To this mixture is added the neat ester 1 (10.0 g, 78.0 mmol), and the mixture is stirred for 3 min at 100 °C. The mixture is cooled to 0 °C with an ice bath. Diethyl ether (Et2O, 100 mL) is added followed by the slow addition of saturated aqueous NaHCO3 (150 mL) with vigorous stirring. Solid NaHCO3 is then cautiously added to the mixture in small portions until foaming subsides. The contents of the flask are transferred to a separatory funnel with an additional quantity (50 mL) of Et2O, the layers are separated, and the aqueous layer is extracted with Et2O (3 × 60 mL). The organic layers are combined, washed sequentially with water (50 mL) and brine (50 mL), dried over anhydrous sodium sulfate, and filtered. The solvent is evaporated under vacuum and the resulting orange colored oil is purified by fractional distillation (68–69 °C/20 mmHg) to give 5.61–5.63 g (65–66 %) of 3,4-dimethylcyclopent-2-enone (2) (Note 3) as a colorless oil.
2. Notes
1.
Crotonic acid was purchased from TCI and was used as received.
Isopropanol was purchased from KANTO CHEMICAL and was used as received.
Phosphorus pentoxide powder, phosphoric acid (85 wt. % solution in water), concentrated sulfuric acid, and benzene were purchased from Wako and were used as received.
Diethyl ether was purchased from Showa-ether and was used as received.
2.
Physical data for
isopropyl (E)-but-2-enoate: IR (neat, cm
−1) 2981, 2940, 1718, 1661;
1H NMR
pdf (500 MHz, CDCl
3) δ: 1.25 (d,
J = 6.4 Hz, 6 H), 1.86 (dd,
J = 7.0, 1.8 Hz, 3H), 5.05 (septet,
J = 6.4 Hz, 1 H), 5.81 (dq,
J = 15.6, 1.8 Hz, 1 H), 6.95 (dq,
J = 15.6, 7.0 Hz 1 H);
13C NMR (125 MHz, CDCl
3) δ: 17.9, 21.9, 67.3, 123.3, 144.1, 166.1. Purity was established by gas chromatographic analysis (Restek Rtx-20 column, flow rate 1 mL/min, temperature 45–280 °C ramped at 60 °C/min, retention time 6.49 min).
3.
Physical data for
3,4-dimethylcyclopent-2-enone: IR (neat, cm
−1) 2965, 2927, 1712, 1684, 1619;
1H NMR
pdf (500 MHz, CDCl
3) δ: 1.19 (d,
J = 7.3 Hz, 3H), 2.00 (dd,
J = 18.6, 2.1 Hz, 1 H), 2.08 (s, 3 H), 2.64 (dd,
J = 18.6, 6.5 Hz, 1 H), 2.81 (ddt,
J = 7.3, 6.5, 2.1 Hz, 1 H), 5.88 (s, 1 H);
13C NMR (125 MHz, CDCl
3) δ: 17.0, 18.8, 38.8, 44.2, 130.3, 182.6, 208.9. Purity was established by gas chromatographic analysis (Restek Rtx-20 column, flow rate 1 mL/min, temperature 120–250 °C ramped at 50 °C/min, retention time 7.87 min).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The transformation of
isopropyl (E)-but-2-enoate to
3,4-dimethylcyclopent-2-en-1-one represents an example of a general rearrangement of esters of α,β-unsaturated acids to cyclopentenones catalyzed by
polyphosphoric acid at elevated temperature. The reaction was first reported by Conia and Leriverend,
2 and presumably takes place via a fragmentation-recombination-Nazarov cyclization pathway, as shown in Scheme 1. A variety of substituted cyclopent-2-en-1-ones can be prepared by this method; esters of
benzoic acid give 1-indanones under the same conditions (see Table 1).
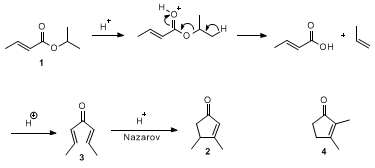
3,4-Dimethylcyclopent-2-en-1-one (
2) is reported to be formed in
60% yield when
hepta-2,5-dien-4-one (
3) is treated with
phosphoric acid and
98% formic acid.
3 However, a later report suggests that the major product from
3 under "Nazarov conditions" (
phosphoric acid and
formic acid at 90 °C) is
2,3-dimethylcyclopent-2-en-1-one (
4),
4 a result in accord with an observation made by Nozaki on Nazarov cyclization of
nona-3,6-dien-5-one, which gave
2,3-diethylcyclopent-2-en-1-one.
5 Exposure of
3 to
fluoro-sulfonic acid at 0 °C gives
2 in
44% yield.
4
3,4-Dimethylcyclopent-2-en-1-one reacts with an alkyllithium reagent and
p-toluenesulfonic acid to give
1,2-dimethylcyclopenta-1,3-diene which, as its lithio or potassio derivative, has been employed in a variety of coupling reactions to produce substituted 1,2-dimethylcyclopentadienes.
6
Bromination of
3,4-dimethylcyclopent-2-en-1-one with
N-bromo-succinimide gives
4-bromo-3,4-dimethylcyclopent-2-en-1-one (Scheme 2).
7 This compound, upon treatment with
triethylamine, generates unstable
3,4-dimethylcyclopentadienone, which undergoes spontaneous self-Diels-Alder cycloaddition to give
endo adduct
5.
83,4-Dimethylcyclopent-2-en-1-one reacts with
sodium azide in the presence of
trifluoroacetic acid to give
5,6-dihydro-4,5-dimethyl-2-pyridone, a formal example of a Schmidt reaction leading to ring expansion.
9
Finally, conjugate addition of
lithium divinylcuprate to
3,4-dimethylcyclopent-2-en-1-one in the presence of
trimethylsilylchloride is stereoselective and yields 3-alkyl-3,4-dimethylcyclopentanone
6 after hydrolysis of the silyl enol ether (Scheme 3).
10
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Crotonic acid:
2-Butenic Acid; (3724-65-0)
Isopropyl (E)-but-2-enoate:
2-Butenoic acid, 1-methylethyl ester, (2E)-; (6284-46-4)
3,4-Dimethylcyclopent-2-enone:
3,4-Dimethyl-2-cyclopenten-1-one; (30434-64-1)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved