Org. Synth. 2006, 83, 70
DOI: 10.15227/orgsyn.083.0070
CATALYTIC ASYMMETRIC ACYLATION OF ALCOHOLS USING A CHIRAL 1,2-DIAMINE DERIVED FROM (S)-PROLINE: (1S,2S)-trans-1-BENZOYLOXY-2-BROMOCYCLOHEXANE
Submitted by Dai Terakado and Takeshi Oriyama
1.
Checked by Jing Zhang, Fangzheng Li, and Marvin J. Miller.
1. Procedure
A. (S)-N-(N-tert-Butoxycarbonylprolyl)dihydroisoindole (2). A dry, 100-mL, two-necked flask equipped with a Teflon-coated magnetic stirring bar and a septum cap is charged with (S)-N-tert-butoxycarbonylproline (5.05 g, 23.5 mmol) (Note 1), dihydroisoindole (2.54 g, 21.3 mmol) (Note 2), and dichloromethane (25 mL) (Note 3) under an argon atmosphere. After cooling to 0 °C with the aid of an ice-water bath, a solution of dicyclohexylcarbodiimide (DCC, 5.1 g, 24.7 mmol) (Note 4) in dichloromethane (20 mL) (Note 3) is added and the reaction mixture is allowed to warm to room temperature while stirring overnight. The mixture is filtered through Celite, concentrated, and purified by column chromatography (Note 5) (ethyl acetate/hexanes:1/3) to afford (S)-N-(N-tert-butoxycarbonylprolyl)dihydroisoindole (4.1 g, 61%) as a white solid (Note 6).
B. (S)-1-Methyl-2-[(dihydroisoindol-2-yl)methyl]pyrrolidine (3). A dry, 200-mL, three-necked, round-bottomed flask equipped with a Teflon-coated magnetic stirring bar, a reflux condenser, a septum cap, and an argon inlet is charged with lithium aluminum hydride (LAH, 0.96 g, 25.3 mmol) in THF (10 mL). After cooling to 0 °C with the aid of an ice-water bath, a solution of (S)-N-(N-tert-butoxycarbonylprolyl)dihydroisoindole (3.96 g, 12.5 mmol) in THF (15 mL) was added under an argon atmosphere. The cooling bath is removed and the reaction mixture is refluxed for 3 h. After cooling the mixture to 0 °C, the reaction is quenched carefully by the slow addition of saturated aqueous sodium sulfate (approx 5 mL). The liquid is decanted away from the precipitate, and the precipitate was washed with THF (2 × 20 mL). The combined liquid is dried over sodium sulfate, filtered and concentrated under reduced pressure. The residue is purified by column chromatography on silica gel (CH2Cl2/MeOH/Et3N:95/5/1), to afford (S)-1-methyl-2- [(dihydroisoindol-2-yl)methyl]pyrrolidine (3) (1.56 g, 58%) (Note 7).
C. (1S,2S)-trans-1-Benzoyloxy-2-bromocyclohexane (4). A 100-mL, two-necked flask equipped with a Teflon-coated magnetic stirring bar and a septum cap is charged with 1 g of molecular sieves 4 Å (Note 8), and flame-dried under reduced pressure. After being allowed to warm to ambient temperature, the apparatus is flushed with argon. The flask is charged with (S)-1-methyl-2-[(dihydroisoindol-2-yl)methyl]pyrrolidine (65 mg, 0.28 mmol) in dichloromethane (5 mL) (Note 3), triethylamine (5.56 g, 55 mmol) (Note 9) in dichloromethane (15 mL), and racemic trans-2-bromocyclohexanol (17.91 g, 100 mmol) (Note 10) in dichloromethane (40 mL) by means of an oven-dried syringe and needle. After cooling to −78 °C by immersion in a dry-ice bath, benzoyl chloride (9.14 g, 65 mmol) (Note 11) in dichloromethane (20 mL) is added slowly over 30 min by means of an oven-dried syringe and needle. The solution is stirred for 3 h at −78 °C, and then quenched with a phosphate buffer (pH 7) (Note 12). The layers are separated and the aqueous layer extracted with diethyl ether (3 × 50 mL). The combined organic phase is washed with water, dried over anhydrous sodium sulfate, filtered, and concentrated under vacuum. The crude products are purified by column chromatography (Note 13) (ethyl acetate/hexanes:1/50) to give (1S, 2S)-trans-1-benzoyloxy-2-bromocyclohexane (14.25 g, 50%) (Note 14) and unreacted alcohol (1R, 2R)-trans-2-bromocyclohexanol (6.60 g, 37%) (Note 15).
2. Notes
1.
The checkers purchased
(S)-N-tert-butoxycarbonylproline from Aldrich Chemical Company.
2.
The checkers purchased
dihydroisoindole (isoindoline) from Aldrich Chemical Company.
3.
Dichloromethane was purchased by the submitters as anhydrous solvent from Kanto Chemical Company, Inc., and used without further purification. The
dichloromethane used by the checkers was purchased from Fisher Scientific and distilled from
CaH2 prior to use.
4.
Dicyclohexylcarbodiimide (DCC) used by the submitters was purchased from Tokyo Kasei Kogyo Co. The checkers used
DCC purchased from Aldrich Chemical Co. The submitters reversed the addition by adding the substrates to the DCC mixture. However, the checkers found that the substrates were incompletely soluble in
methylene chloride and that transfer was then incomplete.
5.
Column chromatography was performed (38 mm × 600 mm column) on Wakogel C-200 that purchased from Wako Chemical Company, Inc. The checkers used
Merck silica gel with a 45 mm × 200 mm column.
6.
The submitters reported
100% yield. The analytical and spectral data of
(S)-N-(N-tert-butoxycarbonylprolyl)dihydroisoindole are as follows:
mp 150–152 °C (decompose),
[α]D24 −18.4 (c 1.0, EtOH). The checkers obtained
[α]D −19.8 (c 1.0, EtOH); The NMR spectra show the presence of two rotameric forms in an approximate 1:1 ratio. Resonances for both rotamers are included in the following characterization data.
1H NMR
pdf (300 MHz, CDCl
3) δ: 1.35 (s, 9 H), 1.46 (s, 9 H), 1.82–2.02 (m, 4 H), 2.08–2.29 (m, 4 H), 3.42–3.68 (m, 4 H), 4.47 (dd, 1 H,
J = 8.1, 4.8 Hz), 4.59 (dd, 1 H,
J = 7.7, 3.3 Hz), 4.72–4.91 (m, 6 H), 4.99 (d, 1 H,
J = 13.6 Hz), 5.18 (d, 1 H,
J = 13.6 Hz), 7.22–7.32 (m, 8 H);
13C NMR (75 MHz, CDCl
3) δ: 23.94 (CH
2), 24.55 (CH
2), 28.50 (CH
3), 28.63 (CH
3), 29.64 (CH
2), 30.49 (CH
2), 46.82 (CH
2), 47.03 (CH
2), 52.35 (CH
2), 52.46 (CH
2), 52.56 (CH
2), 57.55 (CH), 57.89 (CH), 79.69 (C), 79.77 (C), 122.70 (CH), 122.75 (CH), 123.02 (CH), 123.20 (CH), 127.53 (CH), 127.73 (CH), 127.83 (CH), 128.07 (CH), 136.13 (C), 136.26 (C), 136.42 (C), 136.50 (C), 153.92 (C), 154.71 (C), 171.73 (C), 171.89 (C); IR (neat) cm
−1: 2967, 2867, 1694, 1653, 1405, 1358, 1163, 1127, 888, 755; MS (FAB) exact mass, (
m/z) Calcd for C
18H
24N
2O
3 317.1865, Found 317.1862; Anal. Calcd for C
18H
24N
2O
3: C, 68.33; H, 7.65; N, 8.85. Found: C, 68.16; H, 7.60; N, 8.65.
7.
The submitters reported
60% yield after distillation under reduced pressure. The checkers had difficulty with reproducibility using distillation. The yield obtained by the checkers after chromatography varied between
58% and
65%. The checkers used
Merck silica gel with a 45 mm × 200 mm column. The analytical and spectral data of
(S)-1-methyl-2-[(dihydroisoindol-2-yl)methyl]pyrrolidine are as follows: bp
112–114 °C/0.6 mmHg,
[α]D24 −70.4 (c 1.1, EtOH),
1H NMR
pdf (300 MHz, CDCl
3) δ: 1.58–1.84 (m, 3 H), 1.94–2.06 (m, 1 H), 2.16 (dt, 1 H,
J = 9.6, 7.8 Hz), 2.30 (m, 1 H), 2.40 (s, 3 H), 2.62 (dd, 1 H,
J = 11.7, 7.8 Hz), 2.88 (dd, 1 H,
J = 12, 4.8 Hz), 3.03 (m, 1 H), 3.89 (s, 4 H), 7.11 (s, 4 H);
13C NMR (75 MHz, CDCl
3) δ: 22.60 (CH
2), 30.53 (CH
2), 41.35 (CH
3), 57.67 (CH
2), 59.96 (CH
2), 60.94 (CH
2), 64.96 (CH), 122.04 (CH), 126.50 (CH), 140.28 (C); IR (neat) cm
−1: 2938, 2771, 1463, 1149, 742; MS (FAB) exact mass (
m/z) Calcd for C
14H
20N
2 217.1705, Found 217.1701. Anal. Calcd for C
14H
20N
2: C, 77.72; H, 9.32; N, 12.95. Found: C, 77.34; H, 9.53; N, 12.83.
8.
Molecular sieves 4 Å were purchased from Wako Chemical Company, Inc., and dried at 100 °C for 3 h as a powder under reduced pressure before use. The checkers used molecular sieves purchased from Aldrich Chemical Company.
9.
Triethylamine was purchased from Tokyo Kasei Kogyo Co., and distilled before use. The
triethylamine used by the checkers was purchased from Aldrich Chemical Company and was distilled from
CaH2 prior to use.
10.
trans-2-Bromocyclohexanol was prepared from
cyclohexene oxide and
hydrobromic acid according to the following procedure: A 200-mL, round-bottomed flask equipped with a Teflon-coated magnetic stirring bar is charged with
hydrobromic acid (47%, 40 mL, 346 mmol) and cooled at 0 °C by immersion in an ice-water bath.
Cyclohexene oxide (20 mL, 198 mmol) is added dropwise and the mixture is stirred at room temperature for 8 h. After being cooled to 0 °C, the solution is neutralized by addition of saturated aqueous
Na2CO3 (approx 30 mL) (
Caution: slow addition of the Na2CO3 solution is recommended due to excessive bubbling of the solution), and extracted with
diethyl ether (3 × 30 mL). The combined organic phases are dried over anhydrous
sodium sulfate, filtered, concentrated, and distilled under reduced pressure to give
trans-2-bromocyclohexanol (
30.0 g,
85%) (bp
92 °C/11 mmHg).
11.
BzCl was purchased from Tokyo Kasei Kogyo Co., and distilled before use.
12.
The buffer was prepared by dissolving
33.4 g of disodium hydrogenphosphate dodecahydrate and
6.4 g of potassium dihydrogenphosphate into 300 mL of water. The buffer solution was diluted to a final volume of 700 mL and stored in a glass bottle.
13.
The checkers used
Merck silica gel with a 70 mm × 200 mm column.
14.
The checkers obtained
13.65 g (
48%) of the benzoate (
4) and
7.59 g (
42%) of the alcohol. The submitters determined the enantiomeric excess of the benzoate (95%
ee) by HPLC analysis using a Daicel CHIRALCEL OD column (
i-PrOH:hexanes = 1:1000, 1.0 mL/min, 254 nm). The retention times for the
(1S,2S)-trans-1-benzoyloxy-2-bromocyclohexane are 14.6 min ((+)-1
S,2
S) and 16.7 min ((−)-1
R,2
R). The checkers determined the enantiomeric excess of the benzoate to be 90% using a 25 × 0.46 cm Daicel CHIRALPAK AD-H column (
i-PrOH:hexanes = 1:9, 1.0 mL/min, 254 nm). The retention times are 5.05 and 5.31 min. The analytical and spectral data of pure
(1S, 2S)-trans-1-benzoyloxy-2-bromocyclohexane are as follows:
[α]D24 +104.6 (c 1.0, CHCl3, 90.0% ee);
1H NMR
pdf (500 MHz, CDCl
3) δ: 1.35–1.44 (m, 1 H), 1.48–1.57 (m, 2 H), 1.75–1.85 (m, 2 H), 1.91–1.99 (m, 1 H), 2.25–2.33 (m, 1 H), 2.39–2.45 (m, 1 H), 4.16 (m, 1 H), 5.14 (dt, 1 H,
J = 9.0, 4.5 Hz), 7.46 (t, 2 H,
J = 7.5 Hz), 7.57 (m, 1 H), 8.07 (m, 2 H);
13C NMR (125 MHz, CDCl
3) δ: 23.5 (CH
2), 25.6 (CH
2), 31.3 (CH
2), 35.7 (CH
2), 52.8 (CH), 76.5 (CH), 128.5 (CH), 129.9 (CH), 130.4 (C), 133.2 (CH), 165.8 (C); IR (neat) cm
−1: 2941, 1720, 1450, 1274, 1104, 1027, 945, 711; MS (FAB) exact mass (
m/z) Calcd for C
13H
15BrO
2 283.0334, Found 283.0308. The submitters report that the product gave the following elemental anlysis: Anal. Calcd for C
13H
15BrO
2: C, 55.14; H, 5.34. Found: C, 55.05; H, 5.42.
15.
The enantiomeric excess was determined by the submitters to be >99% by HPLC analysis using Daicel CHIRALCEL OD (
i-PrOH:hexanes = 1:1000, 1.0 mL/min, 254 nm) after conversion to the corresponding benzoate. The retention times for the
(1R, 2R)-trans-1-benzoyloxy- 2-bromocyclohexane are 14.3 min ((+)-1
S,2
S) and 16.8 min ((−)-1
R,2
R). The analytical and spectral data of
(1R, 2R)-trans-2-bromocyclohexanol are as follows:
[α]D24 −33.0 (c 1.0, CHCl3, 99.3% ee);
1H NMR
pdf (500 MHz, CDCl
3) δ: 1.22–1.40 (m, 3 H), 1.65–1.70 (m, 1 H), 1.77–1.86 (m, 2 H), 2.10–2.16 (m, 1 H), 2.30–2.36 (m, 1 H), 2.62 (brs, 1 H), 3.60 (dt, 1 H,
J = 9.9, 4.5 Hz), 3.89 (ddd, 1 H,
J = 12.1, 9.5, 4.5 Hz);
13C NMR (125 MHz, CDCl
3) δ: 24.26 (CH
2), 26.8 (CH
2), 33.7 (CH
2), 36.4 (CH
2), 61.96 (CH), 75.4 (CH); IR (neat) cm
−1: 3350, 2835, 1450, 1070, 955.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Asymmetric acylation of alcohols is divided into two types of reactions. These are kinetic resolution of racemic alcohols and desymmetrization of meso-polyols. Although most methods reported so far employ an enzyme such as a lipase or an esterase,
2 some outstanding asymmetric acylations of alcohols by using organocatalysts
3 have recently emerged as reliable alternatives to the well established enzyme-catalyzed reactions.
Kinetic resolution of racemic alcohols via asymmetric acylation has been widely used to construct various useful chiral building blocks in the synthesis of complex natural products.
4 The submitters have demonstrated highly enantioselective desymmetrization of
meso-diols
5 and highly efficient kinetic resolution of racemic secondary alcohols
6 catalyzed by a chiral
1,2-diamine derived from
(S)-proline.
Chiral diamine catalysts can be readily prepared in three steps from
(S)-proline. This type of a
1,2-diamine is frequently used in important and fundamental asymmetric syntheses.
7 This diamine has an advantage in that the non-proline amine portion can be easily modified to include, for example, cyclic
pyrrolidine,
piperidine,
indoline skeletons, or the acyclic benzylmethylamino skeleton. More efficient reactions and higher enantioselectivities during asymmetric acylation of alcohols are achieved by using a chiral diamine containing a
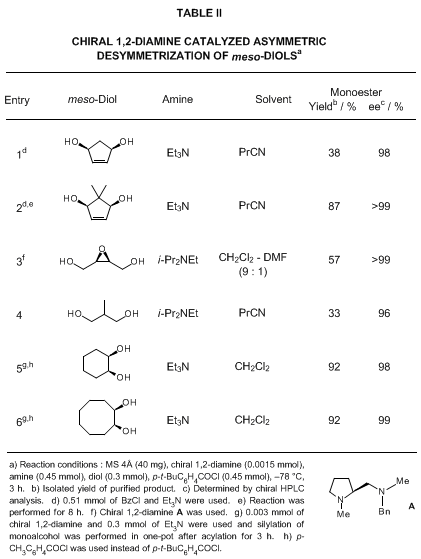
dihydroisoindole or a benzylmethylamino component. Representative results of asymmetric acylation of alcohols catalyzed by chiral 1,2-diamines are shown in Tables I and II.
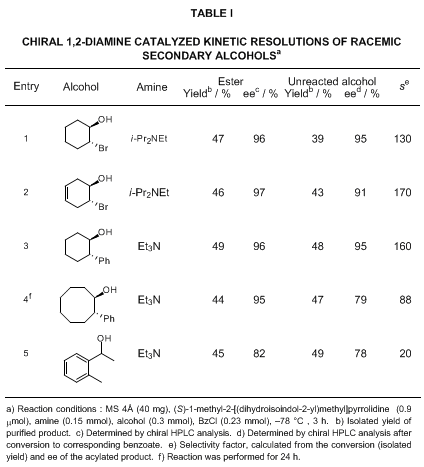
(1S,2S)-trans-1-Benzoyloxy-2-bromocyclohexane is a valuable synthetic precursor for allylic alcohol derivatives. An enantio-enriched allylic benzoate can be provided via β-elimination. Treatment of
4 with
DBN (1,5-diazabicyclo[4.3.0]non-5-ene) in refluxing
toluene gives the corresponding allylic benzoate,
(S)-1-benzoyloxy-2-cyclohexene, without loss of enantio-purity.
6c
We have presented a promising small organocatalyst for asymmetric acylation of various alcohols including racemic secondary alcohols and meso-1,2-, 1,3-, and 1,4-diols. Catalytic asymmetric acylation of alcohols by using a chiral 1,2-diamine has the following distinguishing synthetic features: 1) high enantioselectivity, 2) high efficiency, 3) operational simplicity, 4) widespread applicability, and 5) the absence of a metal.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Di-tert-butyl dicarbonate:
Formic acid, oxydi-, di-tert-butyl ester; Dicarbonic acid, bis(1,1-dimethylethyl)ester; (24424-99-5)
tert-Butoxycarbonyl-L-proline:
1,2-Pyrrolidinedicarboxylic acid, 1-(1,1-dimethylethyl)ester, (S)-tert-Butoxycarbonyl-L-proline (15761-39-4)
Isoindoline, 1,3-Dihydroisoindole:
1H-Isoindole, 2,3-dihydro-; (496-12-8)
Dicyclohexylcarbodiimide:
Carbodiimide, dicyclohexyl-; Cyclohexanamine, N,N'-methanetetraylbis-; (538-75-0)
(S)-N-(N-tert-Butoxycarbonylprolyl)dihydroisoindole (188122-39-6)
(S)-1-Methyl-2-[(dihydroisoindol-2-yl)methyl]pyrrolidine (159497-37-7)
trans-2-Bromocyclohexanol; (2425-33-4)
(1S, 2S)-1-Benzoyloxy-2-bromocyclohexane;
Cyclohexanol, 2-bromo-, benzoate, (1S,2S)-; (222851-77-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved