Org. Synth. 2006, 83, 193
DOI: 10.15227/orgsyn.083.0193
TRIFLUOROMETHANESULFONIMIDE-CATALYZED (2 + 2)-CYCLOADDITION OF SILYL ENOL ETHERS WITH α,β-UNSATURATED ESTERS: 1-(tert-BUTYLDIMETHYLSILOXY)-8-(METHOXYCARBONYL)-6-METHYLBICYCLO[4.2.0]OCTANE
Submitted by Kiyosei Takasu, Takayuki Ishii, Kazato Inanaga, and Masataka Ihara
1.
Checked by Katarzyna Kowalczuk and John A. Ragan.
1. Procedure
A. 1-tert-Butyldimethylsilyloxy-2-Methyl-1-Cyclohexene. A nitrogen- purged, 300-mL, three-necked, round-bottomed flask is equipped with a magnetic stirring bar, a rubber septum, a glass stopper, a temperature probe, and a nitrogen inlet adapter. The flask is charged with 2-methylcyclohexanone (10.0 mL, 82.6 mmol) (Note 1), triethylamine (13.9 mL, 100 mmol) (Note 2), and t-butyldimethylsilyl chloride (TBDMSCl) (15.1 g, 100 mmol) (Note 1). To the flask is added a solution of sodium iodide (15.0 g, 100 mmol) (Note 1) in acetonitrile (100 mL) (Note 3) via syringe over 30 min at ambient temperature. The reaction solution is stirred at ambient temperature for 18 h. The resulting mixture is quenched by addition of saturated sodium bicarbonate solution (100 mL). The mixture is extracted with hexane twice (2 × 200 mL). The combined organic phases are washed with brine (40 mL) and dried over MgSO4, filtered and concentrated at reduced pressure (15–25 mmHg, 25–35 °C) to afford crude product 1 (20.2 g) as a pale yellow oil. This crude product is purified by filtration through a silica gel pad (200 g of silica in a 10-cm diameter fritted glass funnel, height of silica was 20 cm), rinsing with 1 L of hexanes (Note 4) to provide 17.75–17.80 g (95%) of 1 as a colorless oil (Notes 5, 6).
B. 1-(tert-Butyldimethylsilyloxy)-8-(Methoxycarbonyl)-6-Methyl- Bicyclo[4.2.0]Octane. A flame-dried, 300-mL, three-necked, round-bottomed flask is equipped with a magnetic stirring bar, a rubber septum, an internal temperature probe and an argon inlet adapter. The flask is charged with a solution of 1 (4.98 g, 22.0 mmol) in dichloromethane (100 mL) (CH2Cl2) (Note 7) at ambient temperature, and the mixture is cooled in a dry ice-acetone bath to −78 °C while methyl acrylate (1.80 mL, 20.0 mmol) (Note 8) is added. To the mixture is added dropwise a solution of trifluoromethanesulfonimide (80 mM solution; 2.50 mL, 0.20 mmol) in toluene (Notes 9, 10) via syringe over 30 min at −78 °C (Note 11). The mixture is stirred at −78 °C for 30 min (Note 12), and is then quenched with saturated sodium bicarbonate solution (100 mL). The mixture is extracted with methyl tert-butyl ether (MTBE) (2 × 100 mL) (Note 13). The combined organic phases are dried over MgSO4, filtered and concentrated at reduced pressure (15–25 mmHg, 25–35 °C) to afford crude product 2 (7.80 g) as a colorless oil. This crude product is purified by chromatography with 400 g of silica gel (6.5 cm i.d. column, eluent with 50:1 hexane/Et2O) (Note 14) to provide 5.13–5.27 g (82–84%) of the trans-diastereomer of 2 as a colorless oil (Notes 15, 16).
2. Notes
1.
2-Methylcyclohexanone (>98%), t-butyldimethylsilyl chloride (97%), and sodium iodide (>99.5%) were purchased from Aldrich Chemical Company and used as received.
2.
Triethylamine (anhydrous) was purchased from J. T. Baker and used as received.
3.
Acetonitrile (anhydrous, Sure-Seal) was purchased from Aldrich Chemical Company and used as received.
4.
The checkers used 40-µm silica gel for the filtration (J. T. Baker). The submitters purified the product by column chromatography (9.5 cm i.d. column, elution with
hexane) on
400 g of Silica gel 60 N (spherical, neutral, 63–210 mesh), purchased from Kanto Chemical Co. Inc. The
Rf value for
1 is 0.75 (hexane).
5.
The product from step A exhibits the following data: IR (neat): 2928, 2857, 1687, 1348, 1253, 1168, 831 cm
−1;
1H NMR
pdf (400 MHz, CDCl
3) δ: 0.10 (s, 6 H), 0.94 (s, 9 H), 1.51–1.55 (m, 2 H), 1.56 (s, 3 H), 1.60–1.66 (m, 2 H), 1.92–1.95 (m, 2 H), 1.98–2.02 (m, 2 H);
13C NMR
pdf (100 MHz, CDCl
3) δ: −3.6, 16.6, 18.4, 23.2, 24.1, 26.1, 30.5, 30.6, 111.7, 143.2; MS (EI)
m/z: 226 (50%), 169 (80%), 75 (100%); Anal. Calcd for C
13H
26OSi: C, 68.96; H 11.57. Found: C, 68.64; H, 11.64.
6.
A trace amount (<3%) of the regioisomeric silyl enol ether (
1-tert-butyldimethylsiloxy-6-methyl-1-cyclohexene) was observed by
1H NMR spectroscopy. Separation of the isomer is not required for the next reaction.
7.
Dichloromethane (anhydrous, Sure-Seal) was purchased from Aldrich Chemical Company and used as received.
8.
Methyl acrylate (97%) was purchased from Aldrich Chemical Company and used as received. The submitters purchased this material from Tokyo Kasei Kogyo Co., Ltd., and distilled under reduced pressure before use.
9.
Trifluoromethanesulfonimide (95%) was purchased from Aldrich Chemical Company, Inc. A 80 mM solution of
trifluoromethanesulfonimide in
toluene was prepared as follows. A
flame-dried, 50-mL, round-bottomed flask equipped with gas inlet is charged with
trifluoromethanesufonimide (675 mg, 2.40 mmol) under an atmosphere of
argon, and
toluene (anhydrous, Aldrich Sure-Seal) (30 mL) is quickly added under an atmosphere of
argon. The solution can be stored for more than 1 month in the dark at ambient temperature.
10.
Trifluoromethanesulfonimide (neat) is moisture-sensitive and, if possible, should be dissolved in toluene under an
argon atmosphere after a fresh bottle is opened.
11.
The solution temperature inside the reaction vessel was monitored by the internal thermometer and kept within −76 to −78 °C.
12.
Since the (2 + 2)-cycloaddition reaction is reversible, the effects of temperature, reaction time, concentration, and solvent are important. The
trans-isomer of
2 corresponds to the kinetic product. Interestingly, on smaller scale reactions (~100 mg of silyl enol ether), the submitters obtained
trans- and
cis-isomers of
2 in
98% and
1% yields, respectively, under the same conditions.
13.
The submitters performed these extractions with
diethyl ether. The checkers found that MTBE worked equally well for this extraction.
14.
Chromatography was performed on 40-µm silica gel purchased from J. T. Baker. The
Rf values for
trans- and
cis-isomers of
2 are 0.26 and 0.22, respectively (50:1;
hexane/Et
2O), as reported by the submitters. The checkers were unable to cleanly discern the minor isomer on TLC.
15.
The
trans-isomer exhibits the following spectroscopic properties: IR (neat) ν: 2929, 2857, 1734, 1462, 1220, 1095, 834 cm
−1;
1H NMR
pdf (400 MHz, CDCl
3) δ: 0.05 (s, 3 H), 0.10 (s, 3 H), 0.84 (s, 9 H), 1.03 (s, 3 H), 1.14–1.60 (m, 8 H), 1.62–1.64 (m, 1 H), 1.78 (t,
J = 10, 1 H), 3.10 (t,
J = 10, 1 H), 3.62 (s, 3 H);
13C NMR
pdf (100 MHz, CDCl
3) δ: −3.2, −2.9, 18.6, 20.3, 21.8, 24.9, 26.0, 26.9, 32.3, 33.4, 41.3, 48.5, 51.1, 77.6, 173.6; LRMS (EI)
m/z: 255 (M
+ – 57, 100%); Anal. Calcd for C
17H
32O
3Si: C, 65.33; H 10.32. Found: C, 65.22; H, 10.78.
16.
The checkers were unable to isolate a clean sample of the minor isomer, although its presence was confirmed in the crude
1H NMR spectrum (approximately a 10:1 ratio of major/minor isomers, which is in agreement with the submitter's report). The submitters reported the isolation of the
cis-isomer of
2 (
8%), which exhibited the following characterization data: IR (neat): 1746 cm
−1;
1H NMR (400 MHz, CDCl
3) δ: 0.05 (s, 3 H), 0.09 (s, 3 H), 0.85 (s, 9 H), 0.97 (s, 3 H), 1.08–1.24 (m, 1 H), 1.31–1.61 (m, 5 H), 1.75 (m, 2 H), 2.03 (d,
J = 12.2 Hz, 1 H), 2.22 (t,
J = 10.0 Hz, 1 H), 3.16 (t,
J = 7.8 Hz, 1 H), 3.62 (s, 3 H);
13C NMR (100 MHz, CDCl
3) δ: −1.64, −1.58, 18.6, 21.0, 21.6, 24.0, 26.1, 33.4, 33.7, 38.8, 41.0, 43.1, 51.1, 83.7, 172.6; LRMS (EI)
m/z: 255 (M
+ – 57); Anal. Calcd for C
17H
32O
3Si: C, 65.33; H 10.32. Found: C, 65.36; H, 9.98.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
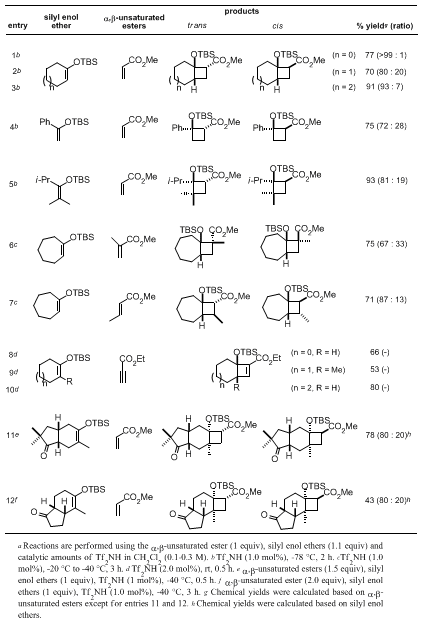
The procedure described here is typical for the catalytic (2 + 2)-cycloaddition reaction of silyl enol ethers with α,β-unsaturated esters to produce multi-substituted silyloxycyclobutanes.
2 Silyl enol ethers are readily available by enol silylation of the corresponding ketones. Previously, we have reported that the (2 + 2)-cycloaddition of silyl enol ethers is catalyzed by a hard Lewis acid such as EtAlCl
2.
3,4,5 Compared with that method, the procedure using
trifluoromethanesulfonimide (Tf2NH) provides high chemical yield and stereoselectivity under practical and environmentally benign conditions, with broader substrate-applicability (Table 1).
We have found the Tf2NH-catalyzed (2 + 2)-cycloadditions of silyl enol ethers with α,β-unsaturated esters are eventually reversible. Although the kinetic product in the reaction possesses the trans-configuration of silyloxy and ester functionalities, longer reaction times or higher reaction temperatures allow the retro (2 + 2)-cycloaddition to occur, leading to the thermodynamically more stable cis-isomer. The isomerization can be monitored by careful TLC analysis.
Yamamoto and coworkers reported that Tf
2NH-catalyzed aldol reactions between TMS enol ethers and aldehydes are promoted by highly reactive,
in situ generated TMSNTf
2.
6 We also observed that a catalytic amount of pre-assembled TBSNTf
2 promoted the (2 + 2)-cycloaddition reactions. Thus, Tf
2NH appears to act similarly as a pre-catalyst to produce the real catalyst TBSNTf
2 through reaction with the
t-butyldimethylsilyl enol ethers. Importantly, decomposition of TBSNTf
2 to form Tf
2NH during the course of these processes is reversed by reaction of the latter with the TBS enol ether substrates. As a result, high turnover numbers are achieved in this catalytic system. Thus, a reaction using a stoichiometric amount of Tf
2NH results in low yields of the desired product and decomposition of the silyl enol ether.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
1-tert-Butyldimethylsilyloxy-2-methyl-1-cyclohexene: Silane, (1,1-dimethyl- ethyl)dimethyl[(2-methyl-1-cyclohexen-1-yl)oxy]-; (20152-33-4)
1-(tert-Butyldimethylsilyloxy)-8-(methoxycarbonyl)-6-methylbicyclo [4.2.0]octane:
Bicyclo[4.2.0]octane-7-carboxylic acid, 6-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-1-methyl-, methyl ester, (1R,6R,7S)-rel-: (657428-75-6)
Trifluoromethanesulfonimide:
Methanesulfonamide, 1,1,1-trifluoro-N- phenyl-N-[(trifluoromethyl)sulfonyl]-; (37595-74-7)
Methyl acrylate:
2-Propenoic acid, methyl ester: (96-33-3)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved