Org. Synth. 2006, 83, 217
DOI: 10.15227/orgsyn.083.0217
IRIDIUM-CATALYZED N-HETEROCYCLIZATION OF PRIMARY AMINES WITH DIOLS: N-BENZYLPIPERIDINE
Submitted by Ken-ichi Fujita
1a
, Youichiro Enoki, and Ryohei Yamaguchi
1b.
Checked by Gustavo Moura-Letts and Dennis P. Curran
1c.
1. Procedure
N-Benzylpiperidine (Note 1). A 100-mL, two-necked, round- bottomed flask fitted with a magnetic stirring bar, a rubber septum, and a reflux condenser with a bubbler-sealed outlet is charged with di-μ-chloro-dichlorobis(η5-pentamethylcyclopentadienyl)diiridium [Cp∗IrCl2]2 (199 mg, 0.25 mmol) (Note 2) and sodium bicarbonate (41 mg, 0.48 mmol) (Note 3) under an argon atmosphere. Addition of 10 mL of toluene (Note 4) by syringe to the flask affords an orange suspension. Benzylamine (10.70 g, 10.91 mL, 99.86 mmol) (Note 5) is added by syringe through the rubber septum over 10 sec. During the addition, the color of the suspension changes to yellow. Then 1,5-pentanediol (10.39 g, 10.45 mL, 99.76 mmol) (Note 5) is added by syringe through the septum over 30 sec. Under an argon flow, the rubber septum is replaced with a glass stopper. The black suspension (Note 6) is heated at reflux in an oil bath (oil bath temperature: 120 °C) for 17.5 h, then the reaction mixture is cooled to room temperature (Note 7). The reflux condenser is removed, and a short-path vacuum distillation apparatus is mounted to the flask. Distillation at 21.0 mmHg yields a fraction boiling between 123–125 °C; the clear, very pale yellow liquid is N-benzylpiperidine (14.20–14.30 g, 81–82% based on 1,5-pentanediol) (Notes 8,9).
2. Notes
1.
This is a modification of a published procedure.
22.
The submitters prepared the iridium complex
di-μ-chloro-dichlorobis(η5-pentamethylcyclopentadienyl)diiridium according to the literature method.
3 They also report that the commercially available
complex from Aldrich Chemical Company, Inc. or Strem Chemicals, Inc. can be substituted. The checkers purchased the catalyst from Strem.
3.
Sodium bicarbonate (EP) was purchased from Wako Pure Chemical Industries, Ltd. (submitters) or Fisher Scientific (checkers) and used as received.
4.
Toluene (GR) was purchased from Wako Pure Chemical Industries, Ltd. (submitters) or Aldrich (checkers) and distilled from
sodium benzophenone ketyl under an argon atmosphere before use.
5.
Benzylamine (GR, >99%) and
1,5-pentanediol (EP, >97%) were purchased from Tokyo Kasei Kogyo Co., Ltd. (submitters) or Aldrich (checkers) and used as received.
6.
The submitters reported that the mixture remained yellow, but the checkers observed that it turned black after about 5 min.
7.
At this time, a small quantity of water was observed in the bottom of the flask.
8.
The submitters reported that the distillate was colorless. The checkers observed that the clear, very pale yellow liquid could be redistilled to provide a clear, colorless liquid, if desired. Alternatively, in a separate run where special care was taken to collect only clear distillate,
13.30 g (
76%) product was obtained.
9.
The spectral data and elemental analysis are as follows:
1H NMR
pdf (300 MHz, CDCl
3) δ: 1.42 (m, 2 H), 1.56 (m, 4 H), 2.36 (m, 4 H), 3.46 (s, 2 H), 7.31–7.23 (m, 5 H);
13C NMR (75 MHz, CDCl
3) δ: 24.3, 25.9, 54.4, 63.8, 126.7, 127.9, 129.0, 138.6; IR (thin film) 3062, 2934, 2852, 2684, 1493, 1369, 1154, 1113 cm
−1; EIMS
m/z: 91(100), 175(95), 98(92), 84(91), 65(75), 176(58). Purity (>99.5%) was assessed by Gas Chromatography (GC) with a retention time (R
t) of 11.16 min: (Agilent 19091Z-413E, HP-1
Methyl siloxane, 0.32 mm × 30 m × 0.25 µm; temperature 50 °C, ramp 10°/min to 350 °C).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
N-Heterocyclic compounds are important intermediates in medicinal chemistry, material chemistry, and synthetic organic chemistry. In particular,
pyrrolidine,
piperidine and
morpholine derivatives are present in large classes of biologically active natural products. For the past several decades, much effort has been devoted to development of efficient methods for the synthesis of these
N-heterocycles. Recently, a variety of transition metal-catalyzed reactions for the synthesis of
N-heterocyclic compounds have been disclosed and reviewed.
4,5 From an environmental point of view,
N-heterocyclization of primary amines with diols is an attractive method because an
N-heterocyclic product can be obtained from easily available starting materials in one pot without generation of wasteful or harmful byproducts (H
2O is the only byproduct). Although some ruthenium-catalyzed systems for
N-heterocyclization of primary amines with diols have been reported, most of them require high reaction temperature (>150 °C) and applicable substrates are rather restricted.
6-9
The method outlined here represents a convenient and environmentally benign
N-heterocyclization of primary amines with diols catalyzed by a Cp∗(C
5Me
5) iridium complex. The reaction can be conducted under relatively mild conditions (reflux in toluene as a solvent), and it does not generate any wasteful byproducts. Additional examples of the [Cp∗IrCl
2]
2-catalyzed
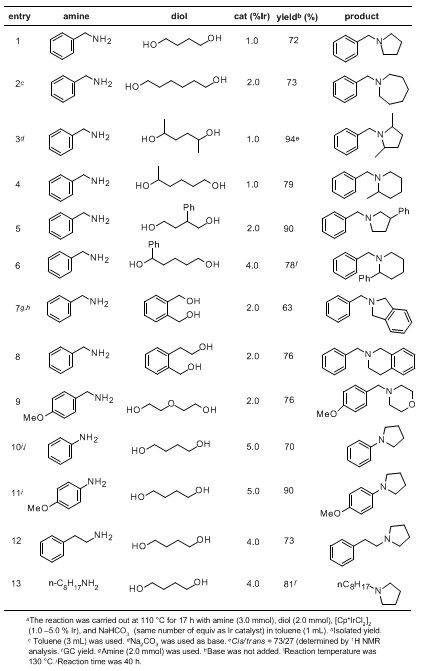
N-heterocyclization of primary amines with diols are shown in the Table.
2 Using this protocol, a variety of pyrrolidine, piperidine, and morpholine derivatives can be synthesized in good to excellent yields. A seven-membered cyclic amine (azepane) can be also synthesized in a satisfactory yield (entry 2).
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Di-μ-chloro-dichlorobis(η5-pentamethylcyclopentadienyl)diiridium [Cp∗IrCl2]2
Iridium, di-μ-chlorodichlorobis[(1,2,3,4,5-η)-1,2,3,4,5-pentamethyl-2,4-cyclopentadien-1-yl]di-; (12354-84-6)
1,5-Pentanediol; (111-29-5)
Benzylamine:
Benzenemethanamine; (100-46-9)
N-Benzylpiperidine:
Piperidine, 1-(phenylmethyl)-; (2905-56-8)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved