Org. Synth. 2013, 90, 338-349
DOI: 10.15227/orgsyn.090.0338
Synthesis of Highly Enantiomerically Enriched Amines by Asymmetric Transfer Hydrogenation of N-(tert-Butylsulfinyl)Imines
Submitted by David Guijarro, Óscar Pablo and Miguel Yus.
1
Checked by Chintan S. Sumaria and Viresh H. Rawal.
1. Procedure
A.
(RS)-N-[1-Phenylethylidene]-2-methylpropane-2-sulfinamide
(3). An oven-dried 100-mL two-necked round-bottomed flask
containing an octagonal Teflon-coated magnetic stirring bar (3.2 x 0.8 cm),
fitted with a vacuum adapter and a rubber septum, is charged with
acetophenone
(1)
(6.01 g, 50.0 mmol, 1.0 equiv) and
(RS)-2-methylpropane-2-sulfinamide
(2) (6.06 g, 50.0 mmol, 1.0 equiv) (Note 1). After applying vacuum followed
by flushing with argon, the stirred mixture is treated with
Ti(OEt)4
(21.3 mL, 22.8
g, 100 mmol, 2.0 equiv) (Note 2), added by syringe.
At this point, the reaction shows an intense yellow color. The rubber septum is
replaced with a glass stopper, and the stirred (700 rpm) mixture is heated so
as to maintain the temperature of the oil bath at 72 °C for 4 h (Note 3), during
which period its color changes to orange. The reaction mixture is cooled to
room temperature using a water bath, diluted with ethyl acetate (40
mL), and then transferred to a 250-mL Erlenmeyer flask
containing a mixture of
ethyl acetate
:brine (25 mL:25
mL). The resulting pale yellow suspension is filtered
through a short plug of Celite (Note 4), which is
washed with
ethyl acetate
(about 1000 mL total) until
no more product elutes (as checked by TLC). The organic layer is concentrated by
rotary evaporation (40-80 mmHg, 28 °C bath temperature) to afford crude
imine 3
as
a yellow oil (Note 5). The crude material is purified by
flash column chromatography (24 x 4.5 cm column containing 160 g of silica
gel) (Note 6). A mixture of
hexanes:ethyl acetate
is
used as the eluent, with a gradient of polarity as
follows:
hexanes:ethyl acetate
100:0 (about 500
mL), 20:1 (about 300 mL), 15:1
(about 300 mL), 9:1 (about 600 mL),
3:2 (about 300 mL), and finally 1:1 until no more
product elutes (about 300 mL) (Notes
7
and
8).
Fractions containing pure product are concentrated by rotary evaporation (40-80
mmHg, 28 °C bath temperature) and the resultant product is then subjected to
high vacuum (5 mmHg, 40 °C bath temperature) to afford
imine 3
(9.54-9.78 g, 85-88% yield) as a yellow solid (Notes
9
and
10).
B.
(R,RS)-N-(1-Phenylethyl)-2-methylpropane-2-sulfinamide
(4). An oven-dried 500-mL two-necked round-bottomed flask is equipped with an octagonal
Teflon-coated magnetic stirring bar (3.8 x 0.9 cm), a condenser topped with a
vacuum adapter with an argon inlet, and a rubber septum. After applying vacuum
and flushing with argon, the flask is charged with
[RuCl2(p-cymene)]2
(490 mg, 0.8 mmol, 0.025 equiv),
2-amino-2-methylpropan-1-ol
(143 mg, 1.6 mmol, 0.05 equiv), and 4 Å MS (3 g) while
maintaining a positive pressure of argon (Notes
11
and
12). The system is again
evacuated and filled with argon [5 cycles vacuum (6 seconds), then argon],
and a positive pressure of argon is maintained throughout the remainder of the
experiment. Dry
isopropyl alcohol
(25 mL) is added
using a syringe (Note 13) and the rubber septum is replaced by a glass stopper.
The stirred (450 rpm) reaction mixture is then placed in a preheated oil bath
(bath temperature 86 °C) for 25 min, resulting in a color change to cherry-red
(Note 14). The oil-bath is replaced with a water-bath (preheated to 50 °C) and
heating is adjusted to maintain the temperature at about 50 °C (Note 15). After
replacing the glass stopper with a rubber septum, a previously prepared
solution of
imine 3
(7.147 g, 32.0 mmol, 1.0 equiv) in
dry
isopropyl alcohol
(160 mL) under argon is added
to the reaction mixture using a cannula (over about
10 min), and the stirring speed is increased to 600 rpm. The temperature of the
system is allowed to stabilize at 50 °C over approximately 5 minutes,
then a previously prepared solution of
potassium tert-butoxide
(449 mg, 4.0 mmol, 0.125 equiv) in dry
isopropyl alcohol
(40 mL) is added to the reaction mixture using a
syringe (Note 16). The resulting brownish mixture is stirred at 50 °C (external
temperature) for 2 h (Note 17). The reaction mixture is cooled to room
temperature using a water bath for 15 min, then filtered through a short plug
of silica gel (Note 18), which is washed with
ethyl acetate
(about 600
mL total) until no more product elutes (as checked by TLC).
In order to ensure the removal of the ruthenium complex, solvents are removed
by rotary evaporation (40-80 mmHg, 28 °C bath temperature) and the residue
is dissolved in 40 mL of
ethyl acetate
and passed
through a short plug of silica gel (Note 19), which is washed with ethyl
acetate (about 500 mL total) until no more product elutes
(as checked by TLC). The filtrate is concentrated by rotary evaporation (40-80
mmHg, 28 °C bath temperature) and then subjected to high vacuum (5 mmHg, 40 °C
bath temperature) to afford pure
sulfinamide 4
(7.20-7.22 g, >99% yield) as
a brown oil (Notes
20
,
21 and 22).
C.
(R)-1-Phenylethanamine
(5). A
250-mL one-necked round-bottomed flask is equipped with an octagonal
Teflon-coated magnetic stirring bar (3.2 x 0.8 cm) and sealed with a septum
fitted with needle connected to a vacuum/nitrogen line. The flask is purged
using a vacuum followed by nitrogen introduction (3 times) and maintained under
a positive pressure of nitrogen. It is then charged with 4 (6.76 g, 30.0 mmol, 1.0 equiv) and dry
methanol
(60 mL) (Note 23) using a syringe. The
solution is cooled to 0-5 °C using an ice-water bath. While stirring, a
2M solution of HCl in
diethyl ether
(75 mL, 150 mmol, 5.0 equiv) is added
by syringe (Note 24). After 5 min, the cooling bath is removed and the reaction
mixture is stirred (600 rpm) at room temperature for 3 h. The solvent is
removed by rotary evaporation (10-80 mmHg, 28 °C bath
temperature). The residue is dissolved in aqueous 2M HCl
solution (100 mL) then extracted with
ethyl acetate
(2 x 80 mL). While the combined organic layers
are discarded, the acidic aqueous layer is cooled to 0-5 °C using an
ice-water bath and treated with aqueous 6M NaOH
solution (100 mL), which is added in portions over 5 min
while stirring, resulting in a pH > 12. The resulting mixture is extracted
with
ethyl acetate
(3 x 50 mL) and the combined
organic layers are washed with H2O (25 mL)
and brine (25 mL). The organic layer is dried
over anhydrous magnesium sulfate (4 g), filtered, then concentrated by rotary
evaporation (40-80 mmHg, 28 °C bath temperature) (Note 25) to afford pure
amine 5
(3.09-3.22 g, 85-88% yield, 95% ee of the (R)-enantiomer)as a pale yellow oil (Notes
26
and
27).
2. Notes
1.
Acetophenone 1
(98%) was purchased from Acros Organics (Ref. No. 102412500).
(RS)-2-Methylpropane-2-sulfinamide 2
(99%) was purchased from AK Scientific, Inc. (Ref. No. 70308). Both compounds were used as received.
2.
Titanium tetraethoxide (33-35% TiO2)
was purchased from Alfa Aesar (Ref. 77142) and used as received.
3. The reaction progress can be monitored by TLC analysis on Merck Silica Gel 60 F
254 glass plates (Ref. 1057150001) and visualization with a UV lamp. Using hexanes:ethyl acetate (7:3) as eluent,
acetophenone 1
has an R
f = 0.64,
sulfinamide 2
has an R
f = 0 and
N-(tert-butylsulfinyl)imine 3
has an R
f = 0.30.
4. The dimensions of the plug of Celite: 8 cm diameter x 2 cm height.
5.
1H NMR analysis of the reaction crude showed that the conversion to
imine 3
was around 90%.
6. Silica gel 60, 230-400 mesh (pore size 60 Å, particle size 40-63 μm) purchased from Sorbent Technologies was used.
7.
Imine 3
has an intense yellow color while the other reagents are colorless, allowing visual monitoring of product elution. When the yellow band reaches the bottom of the column (after about 500 mL of 9:1 hexanes:ethyl acetate) fractions of 20 mL are collected using 30 mL test-tubes until no more product is eluted.
8. The column fractions were checked by TLC analysis as described in
Note 3.
9. The submitters note that if
3
does not solidify after removing the solvent under reduced pressure, the resulting oil solidifies to a yellow solid when kept in a freezer (under argon atmosphere, ca. -25 °C) overnight.
10. The physical and spectroscopic properties of
Imine 3
are as follows:
2
mp 36 °C;
1H NMR
pdf (500 MHz, CDCl
3) δ: 1.33 (s, 9 H), 2.77 (s, 3 H), 7.38-7.52 (m, 3 H), 7.89 (d, J = 7.5 Hz, 2 H);
13C NMR (125 MHz, CDCl
3) δ: 19.7, 22.4 (3 C), 57.3, 127.1 (2 C), 128.3 (2 C), 131.6, 138.6, 176.3; IR (neat, cm
-1) 2979, 1606, 1594, 1572, 1276, 1089, 1067; HRMS (ES+) m/z calcd for C
12H
17NOSNa (M
+ + Na): 246.0929 Found: 246.0927. [α]
20D -14.4 (c 1.09, CH
2Cl
2), {Submitters: [α]
20D -13.0 (c 2.22, CH
2Cl
2)}. Anal. calcd. for C
12H
17NOS: C, 64.53; H, 7.67; N, 6.27. Found: C, 64.76; H, 7.57; N, 6.30.
11. [RuCl
2(
p-cymene)]
2 (98%) was purchased from Strem Chemicals (Ref. 44-0190). 2-Amino-2-methylpropan-1-ol (≥95%) was purchased from Sigma-Aldrich Chemical Co. (Ref. A9199). Both were used as received.
12. Molecular sieves (4 Å; 8-12 mesh beads), purchased from Fischer Scientific (Ref. M514-500), were activated before use by heating at 190 °C for 3 h in a Kugelrohr apparatus under reduced pressure (5 mmHg).
13. Isopropyl alcohol (puriss, absolute over MS, ≥99.5%) (H
2O ≤ 0.005%) was purchased from Sigma-Aldrich Chemical Co. (Ref. 59309) and used as received.
14. The submitters report that ruthenium complex formation was incomplete if the oil-bath temperature was lower than 82 °C.
15. The change from an oil-bath to a water-bath speeds up cooling of the reaction mixture to 50 °C.
16. Potassium
tert-butoxide (≥98%) was purchased from Sigma-Aldrich Chemical Co. (Ref. 156671). It was activated before use by heating for 4 h at 175 °C in a Kugelrohr apparatus under reduced pressure (5 mmHg).
17. The reaction progress can be monitored by TLC analysis on Merck Silica Gel 60 F
254 glass plates (Ref. 1057150001) and visualized with phosphomolibdic acid (5% in EtOH). Using hexanes:ethyl acetate (7:3) as eluent,
Imine 3
has an R
f = 0.30 and the reduction product
4
has an R
f = 0.15.
18. Silica gel 60 plug (230-400 mesh, pore size 60 Å, particle size 40-63 μm): 6 cm diameter x 7 cm height.
19. Silica gel 60 plug (230-400 mesh, pore size 60 Å, particle size 40-63 μm): 5 cm diameter x 5 cm height.
20.
1H NMR and
13C NMR analysis of the isolated crude material indicated that
Imine 3
had cleanly and completely converted to sulfinamide
4
, making further purification unnecessary.
21. The physical and spectroscopic properties of compound
4
are as follows:
3 1H NMR
pdf(500 MHz, CDCl
3) δ: 1.24 (s, 9 H), 1.51 (d, J = 6.5 Hz, 3 H), 3.42 (br s, 1 H), 4.55 (qd, J = 6.5, 3.0 Hz, 1 H), 7.27-7.38 (m, 5 H);
13C NMR
pdf(125 MHz, CDCl
3) δ: 22.6 (3 C), 22.7, 53.9, 55.4, 126.5 (2 C), 127.8, 128.7 (2 C), 144.0; IR (neat, cm
-1) 3214, 2974, 2927, 1455, 1363, 1055, 938, 762, 700; HRMS (ES+)
m/z calcd for C
12H
19NOSNa (M
+ + Na): 248.1085 Found: 248.1087. Checkers: [α]
20D -40.6 (c 1.2, EtOAc); Submitters: [α]
20D -42.5 (c 1.9, EtOAc). Anal. Calcd. For C
12H
19NOS: C, 63.96; H, 8.50; N, 6.22. Found: C, 63.67; H, 8.34; N, 6.29.
22. A 300 mg sample was purified by column chromatography (6 x 1.5 cm column containing 6 g of silica gel). A mixture of hexanes:ethyl acetate was used as eluent, with a gradient of polarity as follows: 7:3 (10 mL), 3:2 (20 mL), 1:1 (20 mL), and 2:3 until no more product elutes (about 50 mL). Compound
4
was isolated by rotary evaporation (276 mg, 92 % recovery).
1H and
13C NMR spectra, as well as [α]
D20, were identical to the crude product (
Note 21
). Anal. Calcd. For C
12H
19NOS: C, 63.96; H, 8.50; N, 6.22. Found: C, 64.03; H, 8.49; N, 6.30.
23. Anhydrous methanol (99.8%) was purchased from Sigma-Aldrich Chemical Co. (Ref. 322415) and used as received.
24. The 2M solution of HCl in diethyl ether was purchased from Alfa Aesar (Ref. H26914) and used as received.
25.
Important: The final product
should not be concentrated to dryness. The concentration should be halted with about 5 mL of liquid remaining. Final solvent removal was carried out by gently blowing a stream of nitrogen or argon over the top of the liquid at atmospheric pressure for 5-10 min, until NMR showed the clean product.
26. The physical and spectroscopic properties of
amine 5
are as follows:
4 1H NMR
pdf(300 MHz, CDCl
3) δ: 1.39 (d, J = 6.5 Hz, 3 H), 1.56 (br s, 2 H), 4.11 (q, J = 6.5 Hz, 1 H), 7.20-7.27 (m, 1 H), 7.30-7.39 (m, 4 H);
13C NMR
pdf(75 MHz, CDCl
3) δ: 25.6, 51.3, 125.6 (2 C), 126.7, 128.4 (2 C), 147.8; IR (neat, cm
-1) 3364, 3029, 2969, 1617, 1559, 1474, 1450, 1376, 1027, 764, 696; HRMS (ES+)
m/z calcd for C
8H
11NNa (M
+ + Na): 144.0789 Found: 144.0780. Submitters: [α]
20D +31.5 (c 2.7, CHCl
3); Aldrich {Ref. 77879, for (R)-5 (99%
ee)}: [α]
20D +33.8 (c 1.2, CHCl
3).
27. The enantiomeric excess of
amine 5
was evaluated as follows. A solution of
5
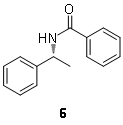
(24 mg, 0.2 mmol) in dichloromethane (5 mL) was cooled to 0-5 °C using an ice-water bath. An aqueous 2M NaOH solution (5 mL) was added followed by benzoyl chloride (46 μL, 0.4 mmol), and the resulting mixture was stirred for 4 h at room temperature. The organic layer was separated and washed with aqueous 2M NaOH solution (2 x 5 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure (40-80 mmHg), to afford the corresponding benzamide
6
(40 mg, 89% yield) as a white solid. Compound
6
was analyzed by HPLC on an Agilent 1100-series instrument equipped with a Chiracel OD-H column (25 x 0.46 cm), using 10% isopropyl alcohol in
n-hexane as eluent, a flow rate of 0.5 mL/min and UV detection at 240 nm. The retention times of the two enantiomers were 27.2 min (
R, 97.4%) and 39.9 min (
S, 2.6%). The racemic amine
rac-
5 was prepared by reductive alkylation of ammonia by acetophenone following a literature procedure
5 and was benzoylated as described above.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The diastereoselective reduction of
N-(
tert-butylsulfinyl)ketimines is among the most useful methods for the preparation of enantiomerically enriched amines. The asymmetric reduction of this type of imine has been performed using boranes,
6a,
6b,
7 sodium or lithium borohydrides,
6,
7,
8 aluminium hydrides,
6b,
7,
8 and diethylzinc in the presence of Ni(acac)
2.
9 The present procedure utilizes a methodology based on asymmetric transfer hydrogenation, which is a very convenient reduction protocol because it avoids the handling of molecular hydrogen or metallic hydrides, among other reasons. Thus, highly enantiomerically enriched α-branched primary amines are obtained in excellent yields by the diastereoselective reduction of a variety of sulfinylimines followed by desulfinylation of the nitrogen atom.
10 The
N-(
tert-butylsulfinyl)imines are prepared by reaction of stoichiometric amounts of the corresponding ketones and 2-methylpropane-2-sulfinamide in the presence of two equivalents of Ti(OEt)
4 under solvent-free conditions.
11 The obtained imines
3
are reduced by asymmetric transfer hydrogenation using isopropyl alcohol as hydrogen source and, as a catalyst, a ruthenium complex bearing a commercially available simple achiral β-aminoalcohol: 2-amino-2-methylpropan-1-ol. This catalyst has been shown to be very versatile, giving good results for the reduction of a variety of aromatic, heteroaromatic and aliphatic sulfinylketimines, including several examples of sterically congested imines. The reduction products
4
are treated with a solution of HCl in a mixture of Et
2O and MeOH to afford the corresponding free primary amines
5
in excellent yields and enantiomeric excesses (Table 1).
Table 1. Other primary amines prepared by this procedure
It is worth noting that very good results are obtained for the reduction of the less reactive aliphatic imines. Our procedure represents a general method for the synthesis of highly enantiomerically enriched aromatic and aliphatic primary amines. The enantiomeric (
S)-amines are also readily accessible with the same optical purity using the same ruthenium catalyst by changing the absolute configuration of the sulphur atom in the starting sulfinamide (see the last example in Table 1).
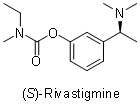
The synthetic utility of some of the obtained α-branched primary amines has already been shown. For instance, (
S)-1-(3-methoxyphenyl)ethanamine has been used as a key intermediate in a short total synthesis of (
S)-Rivastigmine, a cholinesterase inhibitor used for the treatment of moderate dementia in patients with Alzheimer or Parkinson diseases.
12
Appendix
Chemical Abstracts Nomenclature
(Registry Number)
Acetophenone; 1-phenyl-ethanone (1) (98-86-2)
(RS)-2-Methylpropane-2-sulfinamide; [S(R)]-2-methyl-2-propanesulfinamide (2) (196929-78-9)
(RS)-N-[1-Phenylethylidene]-2-methylpropane-2-sulfinamide (3)
(R,RS)-N-(1-Phenylethyl)-2-methylpropane-2-sulfinamide (4)
(R)-1-Phenylethanamine; (R)-α-methyl-benzenemethanamine (5) (3886-69-9)
(R)-N-(1-phenylethyl)benzamide (6)
Titanium(IV) ethoxide; tetraethyl titanate (3087-36-3)
[RuCl2(p-cymene)]2; Di-µ-chlorodichlorobis[(1,2,3,4,5,6-µ)-1-methyl-4-(1-methylethyl)benzene]di-Ruthenium (52462-29-0)
2-Amino-2-methylpropan-1-ol; 2-amino-2-methyl-1-propanol (124-68-5)

|
Miguel Yus was born in Zaragoza (Spain) in 1947, and received his BSc (1969), MSc (1971) and PhD (1973) degrees from the University of Zaragoza. After spending two years as a postdoctoral fellow at the Max Planck Institut für Kohlenforschung in Mülheim, Ruhr he returned to the University of Oviedo where he became associate professor in 1977, being promoted to full professor in 1987. In 1988 he moved to the University of Alicante where he is currently the head of the Organic Synthesis Institute (ISO). His current research interest is focused on the preparation of reactive functionalized organometallic compounds and their use in synthetic organic chemistry and asymmetric catalysis.
|

|
David Guijarro was born in Alicante in 1967. He received his BSc (1990), MSc (1991) and PhD (1994) degrees in Chemistry from the University of Alicante. He did a postdoctoral stay at the University of Uppsala (Sweden) (1995-1997), after which he went back to the University of Alicante, where he has occupied the following academic positions: University College Professor (2000-2003), Associate Professor (2003-2011) and Full Professor (currently). He has been visiting scientist at the University of Warwick (Coventry, UK) (2009). His current research interests are focused on asymmetric synthesis, especially in stereoselective additions of organozinc reagents to carbonyl compounds and imines and in reduction processes of the same type of compounds by hydrogen transfer reactions.
|

|
Óscar Pablo Rodríguez was born in 1985 in Alicante (Spain). He obtained his BSc in Chemistry in 2008 from the University of Alicante with an honorary mention for his academic achievements. In 2008 he started his PhD studies at the same university under the supervision of Professors Miguel Yus and David Guijarro. His current research activities are focused on the asymmetric transfer hydrogenation of activated imines. He is interested in asymmetric synthesis and the development of new synthetic methodologies. He is co-author of 5 publications in international journals.
|

|
Chintan S. Sumaria was born in 1987 in Mumbai, India. He obtained his undergraduate education at the Indian Institute of Technology - Bombay (IIT Bombay), Mumbai, where he carried out undergraduate research in the areas of chemical biology and synthetic methodology. In 2011, he joined the University of Chicago Chemistry Graduate Program and has been working on expanding the scope the Inverse-Electron Demand Diels Alder reactions of azadienes in the research group of Professor Viresh H. Rawal. He has been the recipient of the KVPY scholarship funded by the Department of Science and Technology of the Government of India and the Burjor-Godrej scholarship offered by the Department of Chemistry, IIT Bombay.
|
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved