Allenamides, especially chiral allenamides, are important synthetic intermediates and exist as key structural motifs in many natural products. We recently reported an efficient route to the formation of chiral allenamides that relied upon the isomerization of chiral propargylic oxazolidinones for the introduction of the chiral allenamide functionality.
2 More recently, the stereospecific amidation of optically enriched allenyl iodides using catalytic
copper(I) salt and
N,N-dimethylethylene-diamine as ligands has emerged as a facile strategy to access chiral allenamides
3 (Scheme 1). In recent years, major advances in allenamide chemistry have been concerned with the applications of chiral allenamides to stereoselective synthesis. These advances will be summarized in this article.
(I) CYCLIZATION REACTIONS
The ability of γ-substituted allenamides to undergo cyclization reactions
4 using either stoichiometric TBAF or catalytic PPTS affords stereodivergent syntheses of 2,5-disubstituted dihydrofurans (Scheme 2). The resulting products were then subjected to stereoselective dihydroxylations demonstrating the synthetic utility of chiral allenamides in the stereoselective synthesis of complex molecules.
Cyclization of allenamides can also proceed via a radical mechanism
5 using a combination of AlBN and
n-Bu
3SnH (Scheme 3). The radical addition reactions are highly selective for the
central carbon of the
allene, leading to an efficient preparation of
nitrogen heterocycles such as isoquinolines, and carbocycles such as
indane and
naphthalene derivatives. The
exo-cyclization mode could also be achieved in some cases, leading to the synthesis of isoindoles.
(II) CYCLOADDITION REACTIONS
A) [2π + 1π] CYCLOADDITIONS
The Simmons-Smith cyclopropanations
6 of chiral allenamides provides a viable strategy to obtain optically enriched amido-spiro [2.2] pentanes (Scheme 4). This reaction was efficient with good substrate generality, and represents the most direct synthesis of both chemically and biologically interesting amido-spiro [2.2] pentane systems. However, it suffers from poor diastereoselectivities for unsubstituted chiral allenamides. For a-substituted allenamides, the diastereoselectivity was improved, but both mono- and bis-cyclopropanation products were observed.
B) [4π + 2π] CYCLOADDITIONS
An inverse electron-demand
aza [4
π + 2
π] cycloaddition
7 was observed in the reaction of chiral allenamides with 1-azadienes (Scheme 5). In addition, it was also possible to promote a stereoselective intramolecular normal demand [4
π + 2
π] cycloaddition
8 under thermal conditions without the need for metal catalysts (Scheme 6). Both of these works are applicable for the construction of highly functionalized
aza-sugars and related
nitrogen heterocycles synthesis.
C) [4π + 3π] CYCLOADDITIONS
The first intramolecular [4
π + 3
π] cycloaddition
9 using nitrogen-stabilized chiral oxyallyl cations
10 was observed in the epoxidation of
N-tethered allenamides (Scheme 7). The origin of the high selectivities for the oxyallyl cycloadditions was postulated to originate from structure of the nitrogen-stabilized chiral oxyallyl cation intermediate. This strategy was expanded to include the chemoselective epoxidation of allenamides tethered to either α or γ carbon atom of dienes
11 followed by the tandem [4
π + 3
π] cycloaddition step (Scheme 8).
A highly enantioselective version of this [4
π + 3
π] cycloaddition was promoted by catalytic amounts of a chiral Lewis acid complex generated from Cu(OTf)
2 and
C2-symmetric bisoxazolines as ligands.
12 High enantioselectivities were obtained when either unsubstituted or substituted furans were employed as the dienes (Scheme 9).
Mechanistically, it had been rationalized that the observed stereoselectivity for the intermolecular [4π + 3π] cycloaddition between nitrogen-stabilized oxyallyl cation and furan is a result of the furan approaching in a favorable endo manner from the less hindered bottom or endo-I face of the oxyallyl cation. This preference can be further enhanced with a bidentate metal cation such as Zn that can chelate to both the oxyallyl oxygen atom and the oxazolidinone carbonyl oxygen (Scheme 10).
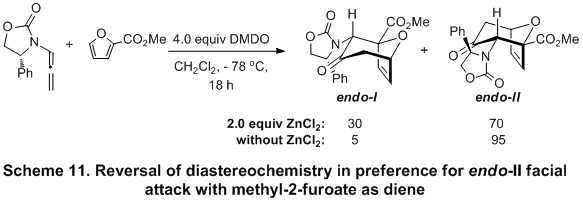
However, an unexpected reversal of diastereoselectivity was observed when methyl 2-furoate was employed as the diene with nitrogen-stabilized oxyallyl cation.
13 This intriguing reversal in favor of the
endo-II cycloaddition pathway is likely a result of the transition state minimizing the dipole interaction between the oxyallyl cation and ester carbonyl of methyl 2-furoate (Scheme 11).
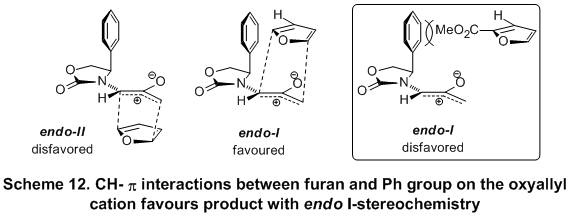
Based on density functional theory calculations, it was further established that the stereo-induction for the [4
π + 3
π] cycloaddition between oxyallyl cation and unsubstituted
furan is due to the stabilizing CH-p interactions between the incoming
furan and the Ph group on the oxyallyl isomer (Scheme 12). These CH-p interactions cause the cycloaddition to take place preferentially via the morecrowded face of the oxazolidinone to afford the product with the stereochemistry resulting from the addition via
endo I-face.
14 In the case of methyl-2-furoate, the reversal of stereoselectivity can be ascribed to the repulsive interactions between the Ph group and the 2-COOMe group which outweighs the stabilizing effect of the CH-p interactions. As such, cycloaddition takes place preferentially through the less crowded transition state affording a product with stereochemistry that can be explained by
endo II-facial attack.
This [4
π + 3
π] cycloaddition reaction was further expanded to include the reactions between oxazolidinone-substituted oxyallyl groups and unsymmetrically substituted furans.
15 Syn regioselectivity was observed when the
furan has a 2-Me or 2-COOR substituent, while
anti regioselectivity is obtained with a 3-Me or 3-COOR group (Scheme 13).
Finally, a practical and diastereoselective intramolecular [4
π + 3
π] cycloaddition of
N-sulfonyl substituted oxyallyl cations with furans was also reported (Scheme 14). Selectivity is found to depend on the tethering length as well as the stability of the oxyallyl cation intermediate, whether generated from
N-carbamoyl- or
N-sulfonyl-substituted allenamides.
16 The use of chiral
N-sulfonyl-substituted allenamide provided minimal diastereoselectivity in the cycloaddition, while high diastereoselectivity can be achieved with a stereocenter present on the tether.
III. ISOMERIZATION REACTIONS
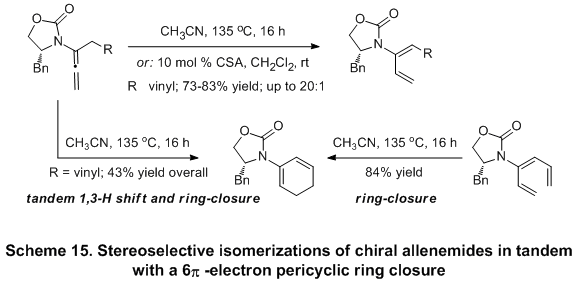
The ability of allenamides to undergo regio- and stereoselective 1,3-hydrogen shift under both acidic and thermal conditions, leads to the
de novo preparations of 2-amido-dienes.
17 This process could be rendered in tandem with a 6
π -electron pericyclic ring closure to access cyclic 2-amido-dienes in good overall yields directly from the respective allenamides (Scheme 15). Further broadening of the scope of this isomerization reaction leads to the development of a new torquoselective ring-closure of chiral amide-substituted 1,3,5-hexatrienes and its application in tandem with [4
π + 2
π] cycloaddition<
18 (Scheme 16). The trienes were derived via either a 1,3-hydrogen or 1,3-H-1,7-hydrogen shift of a-substituted allenamides, and the entire sequence through the [4
π + 2
π] cycloaddition could be promoted in tandem.
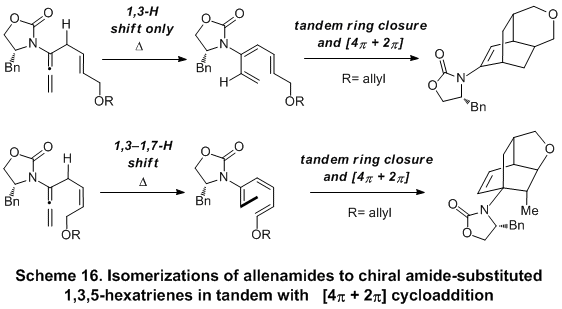
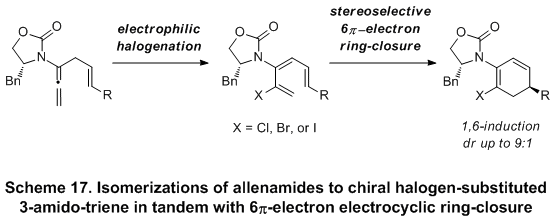
The efforts reported thus far unveiled an invaluable opportunity not only to develop a new and attractive template for conducting stereoselective 6p-electrocyclic ring-closures, but also to achieve a highly challenging 1,6-asymmetric induction. Indeed, a diastereoselective 6
π-electrocyclic ring-closure employing halogen-substituted 3-amidotrienes via a 1,6-remote asymmetric induction was subsequently reported
19 (Scheme 17). This new asymmetric manifold for pericyclic ring-closure further underscores the significance of the allenamide chemistry.
In addition, 1,3-hydrogen shifts using allenamide
20 is also applicable to the preparation of acyclic 2-amido-dienes and 3-amido-trienes. Additionally, 6
π-electron electrocyclic ring-closure could be carried out using 3-amido-trienes to afford cyclic 2-amido-dienes, and such electrocyclic ring-closure could be rendered in tandem with the 1,3-hydrogen shift, thereby constituting a facile construction of synthetically rare cyclic 2-amido-dienes (Scheme 18).
Finally, a new approach to Oppolzer's intramolecular Diels-Alder cycloaddition [IMDA] through γ-isomerization
21 of readily available
N-tethered allenamides is described. These IMDA reactions are carried out in tandem with the allenamide isomerization or 1,3-hydrogen shift, leading to complex
nitrogen heterocycles in a highly stereoselective manner (Scheme 19).
(IV) NATURAL PRODUCT SYNTHESIS
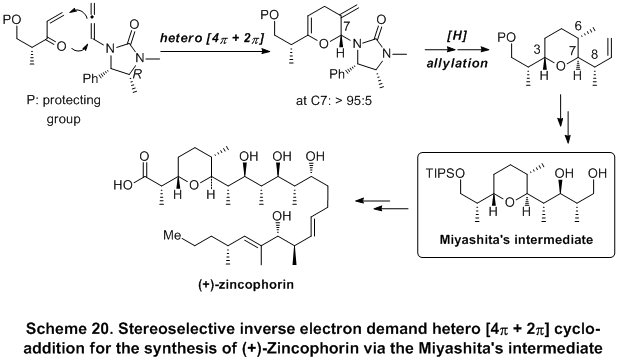
The synthetic usefulness of the stereoselective inverse electron-demand [4
π + 2
π] cycloaddition using chiral allenamide was demonstrated in the formal synthesis of
(+)-zincophorin. This effort describes the preparation of the
Miyashita's intermediate which features the first synthetic application of a stereoselective inverse electron demand hetero [4
π + 2
π] cycloaddition of a chiral allenamide and an interesting
urea directed Stork-Crabtree hydrogenation (Scheme 20).
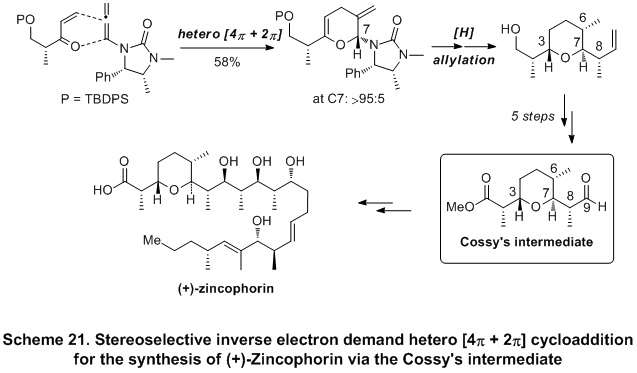
22 This approach was subsequently applied for the construction of the Cossy's C1-C9 subunit of
(+)-zincophorin23, which also led to the observation of an unusual
urea directed Stork-Crabtree hydrogenation (Scheme 21). It is noteworthy that these works represent the first application of chiral allenamides in natural product synthesis.
Finally, a highly stereoselective [4
π + 3
π] cycloaddition of
N-substituted pyrroles with allenamide-derived nitrogen-stabilized chiral oxyallyl cations was reported which could serve as a useful approach towards the construction of the
aza-tricyclic core of
parvineostemonine24 (Scheme 22).
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved