Org. Synth. 2021, 98, 242-262
DOI: 10.15227/orgsyn.098.0242
Synthesis of Tetraaryl-, Pentaaryl-, and Hexaaryl-1,4-dihydropyrrolo[3,2-b]pyrroles
Maciej Krzeszewski, Mariusz Tasior, Marek Grzybowski, and Daniel T. Gryko*
1Checked by Shu Nakamura, Koichi Hagiwara, and Masayuki Inoue
1. Procedure (Note 1)
A. 1,4-Bis(4-(tert-butyl)phenyl)-2,5-bis(4-cyanophenyl)-1,4-dihydropyrrolo[3,2-b]pyrrole (1). A three-necked, 200 mL round-bottomed flask (main neck 29/32 joint, side neck 15/25) is equipped with a 5 cm Teflon-coated football shaped magnetic stir bar. All three necks are left open to the atmosphere. Acetic acid (50 mL) (Note 2) and toluene (50 mL) (Note 3) are poured into the flask via a glass funnel. 4-Cyanobenzaldehyde (7.87 g, 60.0 mmol, 2 equiv) (Note 4) is dissolved in the solvent mixture, then 4-tert-butylaniline (9.0 g, 9.6 mL, 60 mmol, 2 equiv) (Note 5) is added via a plastic syringe (Figure 1A). The flask is immersed in a silicone oil bath preheated to 50 °C (Notes 6 and 7).
Figure 1. A) Addition of 4-tert-butylaniline via a syringe into dissolved 4-cyanobenzaldehyde. B) Solution stirred for 30 min at 50 °C. C) Solution color change after addition of Fe(OTs)3· 6H2O.
The clear yellow solution is stirred for 30 min under air atmosphere (Figure 1B).
Iron(III) p-toluenesulfonate hexahydrate (1.22 g, 1.80 mmol, 6 mol%) (
Note 8) is added, causing immediate reddening of the solution (Figure 1C). Immediately after addition of
Iron(III) p-toluenesulfonate hexahydrate,
butane-2,3-dione (2.6 g, 2.6 mL, 30 mmol, 1 equiv) is added dropwise over 3 min
via a plastic syringe, causing blackening of the solution (
Note 9) (Figure 2A). The mixture is heated at 50 °C. After ca. 10 min, the reaction mixture turns orange and a yellow precipitate is formed (Figure 2B). The suspension is vigorously stirred for 16 h at 50 °C with all three necks uncapped to provide access of the oxygen from the air (
Note 10) (Figure 2C). The oil bath is removed to allow the reaction mixture to cool to room temperature over 15 min (
Note 11). The resulting dark mixture with yellow precipitate (Figure 3A) is treated with
methanol (150 mL) (
Note 12) and vacuum filtered through a Kiriyama-funnel (S-60, filter paper: No.5B, 60 mmφ). The filtered solid is rinsed with
methanol (150 mL) (Figure 3B), followed by
diethyl ether (50 mL) (
Note 13) (Figure 3C). The resulting solid is dried under vacuum (4.8 mmHg) at 95 °C for 3 h (Figure 4A) to give the tetraarylpyrrolo[3,2-
b]pyrrole (TAPP, compound
1) as a yellow solid (10.21 g, 17.82 mmol, 59%) (Notes
14,
15, and
16) (Figure 4B).
Figure 2. A) Dropwise addition of butane-2,3-dione via a syringe.
B) Precipitate formed after ca. 10 min after dione addition. C) Suspension after 1 hour stirring.
Figure 3. A) Reaction mixture after 16 h. B) Appearance after rinsing with methanol. C) Appearance after rinsing with diethyl ether (photos provided by submitters).
Figure 4. A) Drying set-up. B) Pure and dry TAPP (compound 1) (photos provided by submitters).
B. [1,4-Bis(diphenylphosphino)butane](η3-allyl)palladium (II) chloride (PdCl(C3H5)(dppb)) (Note 17). A 30 mL Schlenk flask (15/25 joint) is equipped with a 2 cm Teflon-coated football shaped magnetic stir bar. The flask is connected to an argon/vacuum line and the reaction setup is dried under vacuum (4.8 mmHg) by heating with a heat gun and allowed to cool to room temperature. The reaction set-up is backfilled with argon and the argon atmosphere is maintained throughout the reaction using an argon manifold system. The flask is charged with allylpalladium chloride dimer ([Pd(C3H5)Cl]2, 200 mg, 0.547 mmol) (Note 18) and 1,4-bis(diphenylphosphino)butane (dppb, 460 mg, 1.08 mmol) (Note 19). The reaction set-up is evacuated and backfilled with argon three times. Anhydrous dichloromethane (10 mL) (Note 20) is added and the flask is immersed in the silicone oil bath preheated to 25 °C. The solution is stirred at 25 °C for 30 min. The initial yellow solution (Figure 5A) changes color to red in ca. 15 min (Figure 5B). The solvent is removed in vacuum (4.8 mmHg at room temperature) and dried under vacuum (4.8 mmHg at room temperature for 3 h) to give a pinkish powder, which is used without further purification (Note 21) (Figure 5C-D).
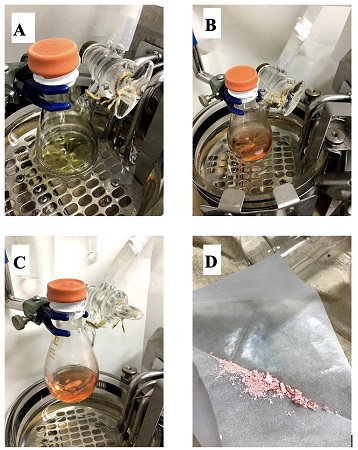
Figure 5. A) Appearance of reaction mixture immediately after addition of dichloromethane. B) Solution stirred for 15 min at 25 °C C) Drying set-up. D) Appearance of the crude PdCl(C3H5)(dppb) catalyst.
C. 1,4-Bis(4-(tert-butyl)phenyl)-2,5-bis(4-cyanophenyl)-3,6-bis(4-nitro-phenyl)-1,4-dihydropyrrolo[3,2-b]pyrrole (2). A three-necked, 500 mL round-bottomed flask (main neck 29/32 joint, side neck 15/25 joint) (flask A) is equipped with a 5 cm Teflon-coated football shaped magnetic stir bar. A 29/32 three-way stop cock is attached to the main neck of the flask and sealed with Teflon tape. The flask is connected to an argon/vacuum line and the reaction setup is dried under vacuum (4.8 mmHg) by heating with a heat gun and allowed to cool to room temperature. Next, the reaction set-up is backfilled with argon and the flask is charged with 1 (5.73 g, 10.0 mmol, 1 equiv), 1-bromo-4-nitrobenzene (12.1 g, 60.0 mmol, 6 equiv) (Note 22), PdCl(C3H5)(dppb) (0.60 g, 0.98 mmol, 10 mol%) and potassium acetate (5.30 g, 54.0 mmol, 5.4 equiv) (Note 23) while purging the flask with argon. The reaction set-up is evacuated and backfilled with argon three times and sealed with a rubber septum. Dry N,N-dimethylacetamide (DMA, 250 mL) (Note 24) is added to flask A via a cannula from a three-necked, 500 mL round-bottomed flask equipped with a three-way stopcock and sealed with rubber septa and Teflon tape) (flask B) with the constant argon flow (Figure 6A). The three-way stopcock of the flask A is closed after solvent addition. The cannula is removed and the flask A is immersed in the silicone oil bath preheated to 150 °C (Note 25).

Figure 6. A) Cannulation of DMA from flask B to reaction flask A. B) Initial color of the solution. C) Appearance after 30 min. D) Appearance after 90 min.
Gradual color change of the solution from yellow through green to black is apparent (Figures 6B-D). The solution turns black completely in ca. 90 min. The mixture is stirred at 150 °C for 24 h. After cooling to room temperature over 30 min, the stir bar is removed and the resulting reaction mixture is transferred to 1 L round-bottomed flask, which is rinsed with ethyl acetate (60 mL) that is added to the reaction mixture (Note 26). The reaction mixture is concentrated on a rotary evaporator (70 °C, 10 mmHg) and dried under vacuum (4.8 mmHg, 30 min, room temperature). After evaporation of the reaction mixture, the tarry-black residue (Figure 7A) is dissolved in dichloromethane (250 mL) and transferred to the 1L separatory funnel. Organic layer is washed with distilled water (2 x 250 mL), brine (250 mL) and dried over anhydrous MgSO4 (30 g) (Note 27) for 2 h. The drying agent is filtered off through a cotton plug and rinsed with dichloromethane (150 mL). The filtrate is concentrated on a rotary evaporator (40 °C, 600 to 70 mmHg) to dryness (Figure 7B) and acetonitrile (30 mL) (Note 28) is poured onto the residue. After stirring for 30 min, yellow precipitate formed is vacuum filtered through a Kiriyama-funnel (S-60, filter paper: No.5B, 60 mmφ) and rinsed with acetonitrile (20 mL) (Figures 7C and 10) (Notes 29 and 30).

Figure 7. A) Appearance of the crude reaction mixture after evaporation. B) Appearance of the concentrated organic phase after liquid-liquid extraction. C) Filtration set-up after rinsing with acetonitrile (photos provided by submitters).
Collected solid is dissolved in
dichloromethane (125 mL), and silica gel (50 g) (
Note 31) is added to the solution. The suspension is concentrated on a rotary evaporator (40 °C, 540 to 70 mmHg) to dryness. The resulting solid is purified by a gradient column chromatography on silica gel using
hexane/
dichloromethane mixture as an eluent (Figures 8A and 11) (Notes
32,
33, and
34). Fractions containing solely the desired product are collected, combined, evaporated and dried under vacuum (4.8 mmHg, 30 min, room temperature) (Figure 8B). The solid material is treated with
diethyl ether (25 mL) and vacuum filtered through a Kiriyama-funnel (S-60, filter paper: No.5B, 60 mmφ) and washed with
diethyl ether (25 mL) (Figure 8C). The yellow crystals are collected, dried under vacuum (4.8 mmHg) at 85 °C for 1 hour to give desired hexaarylpyrrolopyrrole (compound
2) as a yellow solid (4.98 g, 6.11 mmol, 61%) (Figure 8D) (
Note 35).
Figure 8. A) Appearance of a column chromatography in progress. B) Drying set-up for combined fractions 13-24. C) Filtration set-up after rinsing with diethyl ether. D) Appearance of the final hexaarylpyrrolo[3,2-b]pyrrole (compound 2).
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
acetic acid,
toluene,
4-cyanobenzaldehyde,
4-tert-butylaniline,
iron(III) p-toluenesulfonate hexahydrate,
butane-2,3-dione,
methanol,
diethyl ether,
allylpalladium chloride dimer,
1,4-bis(diphenylphosphino)butane,
dichloromethane,
1-bromo-4-nitrobenzene,
potassium acetate,
N,N-dimethylacetamide,
ethyl acetate,
magnesium sulfate,
acetonitrile, silica gel, hexanes, and
dibromomethane.
2.
Acetic acid (>99.5%) was purchased from Chempur (
http://en.chempur.pl/) and was used as received (submitters).
Acetic acid (>99.7%) was purchased from Nacalai Tesque, Inc. and was used as received (checkers).
3.
Toluene (>99.5%) was purchased from POCh (
http://www.english.poch.com.pl/) and was used as received (submitters).
Toluene (>99.5%) was purchased from Nacalai Tesque, Inc. and was used as received (checkers).
4.
4-Cyanobenzaldehyde (99%) was purchased from Fluorochem and was used as received.
5.
4-tert-Butylaniline (98%) was purchased from Fluorochem and was used as received (submitters).
4-tert-Butylaniline (98%) was purchased from Tokyo Chemical Industry Co., Ltd. and was used as received (checkers).
6. A stir plate was purchased from Heidolph Instruments GmbH & Co. KG. It has an input power of 230-240 V (50-60 Hz, 825 W), and the stirring range is from 100 rpm to 1400 rpm. Unless specified differently, 300 rpm was used for stirring (submitters). A stir plate was purchased from Yazawa-Kagaku. It has an input power of 100 V (50-60 Hz, 10 W), and the stirring range is from 100 rpm to 1500 rpm. Unless specified differently, 900 rpm was used for stirring (checkers).
7. The silicone oil for the oil bath was purchased from Carl Roth. The boiling point is over 205 °C. Unless specified differently, the oil in the oil bath should cover the reaction mixture in the reaction flask while heating. Unless reported, the temperatures throughout this manuscript refer to temperatures of oil in the oil baths (submitters). The silicone oil for the oil bath was purchased from Shin-Etsu Chemical Co., Ltd. The flash point is over 300 °C (checkers).
8.
Fe(OTs)3· 6H2O (technical grade) was purchased from Sigma Aldrich and was used as received.
9.
Butane-2,3-dione (99%) was purchased from Alfa Aesar and was used as received.
10. If the precipitate prevented stirring within 15 min of heating, it needed to be crushed manually to facilitate the stirring. Efficient stirring providing access of an oxygen in the whole reaction's volume is crucial for the large-scale syntheses of tetraarylpyrrolo[3,2-
b]pyrroles (see ref 8).
11. The room temperature throughout this manuscript refers to temperature between 22 °C and 25 °C.
12.
Methanol (>99.0%) was purchased from POCh and was used as received (submitters).
Methanol (>99.0%) was purchased from Nacalai Tesque, Inc. and was used as received (checkers).
13.
Diethyl ether (>99.5%) was purchased from POCh and was used as received (submitters).
Diethyl ether (>99.5%) was purchased from Nacalai Tesque, Inc. and was used as received (checkers).
14. When the reaction was carried out on a 15 mmol scale, 5.56 g (65%) of the product was obtained.
15. TLC analysis of the product
1 is shown below. The R
f value of the product
1 in
hexane/
dichloromethane (1/4, v/v) was 0.74. The spot of the product can be viewed on silica gel 60 F
254 plates (TLC Silica gel 60 F
254, purchased from Merck KGaA) with UV light (254 nm).
Figure 9. TLC analysis of Step A
16.
1,4-Bis(4-(tert-butyl)phenyl)-2,5-bis(4-cyanophenyl)-1,4-dihydropyrrolo[3,2-b]pyrrole (1) has the following physical properties: mp 343.5-354.5 °C (dec.); IR (film): 2958, 2868, 2224, 1599, 1518, 1459, 1380, 1362, 1171, 766 cm
-1;
1H NMR
pdf (500 MHz, CDCl
3) δ: 1.36 (s, 18H), 6.49 (s, 2H); 7.18 (AA'XX', d,
J = 8.6 Hz, 4H), 7.28 (AA'XX', d,
J = 8.6 Hz, 4H), 7.42 (AA'XX', d,
J = 8.6 Hz, 4H), 7.47 (AA'XX', d,
J = 8.6 Hz, 4H);
13C NMR
pdf (125 MHz, CDCl
3) δ: 31.4, 34.6, 96.1, 109.0, 119.2, 124.8, 126.4, 127.8, 131.9, 133.4, 135.0, 136.6, 137.7, 149.7; HRMS (ESI)
m/z calcd for C
40H
36N
4Na
+ [M + Na]
+ 595.2832, found 595.2822; Anal. calcd for C
40H
36N
4: C, 83.88; H, 6.34; N, 9.78. Found: C, 83.77; H, 6.49; N, 9.70.
17.
PdCl(C3H5)(dppb) catalyst was prepared according to the procedure reported by Doucet et. al. in
ChemCatChem 2013,
5, 255-262 (Ref. 12c).
18.
Allylpalladium chloride dimer (>97%) was purchased from Tokyo Chemical Industry Co., Ltd. and was used as received.
19.
1,4-Bis(diphenylphosphino)butane (>98%) was purchased from Tokyo Chemical Industry Co., Ltd. and was used as received.
20.
Dichloromethane (99%) was purchased from Linegal Chemicals (
http://www.linegal.com.pl/en/) and was used as received. For water-sensitive reactions it was dried using Solvent Purification System from MBraun (
https://www.mbraun.com/us/) (submitters).
Dichloromethane (>99.5%) was purchased from FUJIFILM Wako Pure Chemical Corporation and purified by Glass Contour solvent dispensing system (Nikko Hansen & Co., Ltd.) when it was used for Step B. Otherwise,
dichloromethane (>99%) was purchased from Kanto Chemical Co., Inc. and was used as received (checkers).
21. NMR analysis of the crude palladium catalyst (
PdCl(C3H5)(dppb)):
1H NMR
pdf (500 MHz, CDCl
3) δ: 1.71-1.88 (m, 4H), 2.54-3.11 (m, 4H), 3.74 (bs, 4H), 5.60 (quint,
J = 10.3 Hz,1H), 7.35-7.60 (m, 20H);
13C NMR
pdf (125 MHz, CDCl
3) δ: 23.3 (m), 27.2 (t), 74.6 (t), 120.4, 128.8 (t), 130.5, 132.7;
31P NMR
pdf (202 MHz, CDCl
3) δ: 18.5 (s).
22.
1-Bromo-4-nitrobenzene (99%) was purchased from Sigma Aldrich and was used as received.
23. Anhydrous
KOAc was purchased from Chempur and was additionally dried under vacuum (0.80 mmHg) at 150 °C for 2 h prior to use (submitters). Anhydrous
KOAc (>99%) was purchased from Sigma Aldrich and was additionally dried under vacuum (4.8 mmHg) at 150 °C for 11 h prior to use (checkers).
24.
N,N-Dimethylacetamide (
DMA), (>99%) was purchased from Sigma Aldrich and was additionally dried with molecular sieves 4Å (50 g) for 16 h, which were heated at 300 °C for 6 h in a furnace (submitters).
N,N-Dimethylacetamide (
DMA), (>99%) was purchased from Sigma Aldrich and was additionally dried in flask B with molecular sieves 4Å (50 g) for 12 h, which were heated by microwave oven (500W, 3 x 90 seconds) (checkers).
25. The reaction was performed under argon atmosphere in the flask sealed with septum. To prevent over-pressurization, after stirring for 1 h at 150 °C the rubber septum was equipped with a needle adapter and the three-way stop cock connected to the argon/vacuum line was opened to faciliate constant argon flow. The flask was flushed with argon for 30 seconds, after which the three-way stopcock was closed and the needle adapter removed.
26.
Ethyl acetate (>99%) was purchased from Kanto Chemical Co., Inc. and was used as received (checkers).
27. Anhydrous
MgSO4 was purchased from Chempur and was used as received (submitters). Anhydrous
MgSO4 was purchased from Tokyo Chemical Industry Co., Ltd. and was used as received (checkers).
28.
Acetonitrile (99.9%) was purchased from POCh and was used as received (submitters).
Acetonitrile (99%) was purchased from Nacalai Tesque, Inc. and was used as received (checkers).
29. In order to get better separation of the products on the column chromatography by preventing overloading, the crude reaction mixture was treated with
acetonitrile.
30. TLC analysis of the crude product before column chromatography is shown below. The R
f value of the product
2 in
hexane/
dichloromethane (1/4, v/v) was 0.31. The spot of the product can be viewed on silica gel 60 F
254 plates (TLC Silica gel 60 F
254, purchased from Merck KGaA) with UV light (254 nm).
Figure 10. TLC analysis of Step C before column chromatography
31. Silica gel (Silica gel 60, 0.040-0.063 mm) was purchased from Merck KGaA and was used as received.
32. Hexanes (95%) were purchased from Linegal Chemicals and distilled prior to use, collecting fraction boiling at 68 °C (submitters).
Hexane (>95%) was purchased from Kanto Chemical Co., Inc. and was used as received (checkers).
33. Flash column chromatography was performed using 950 g of silica gel (Silica gel 60, 0.040-0.063 mm, purchased from Merck KGaA). It was wet packed (9.5 cm in diameter, bed height = 32 cm) using
hexane/
dichloromethane (2/3, v/v) and the silica gel with the crude material adsorbed was loaded onto the column. Sand with 2 cm minimum height was added to the bottom and to the top of the column (sand was used to assist packing). Chromatography reservoir (500 mL, 29/32 joint) was attached. The column was eluted with 4 L of
hexane/
dichloromethane (2/3, v/v), then 1.5 L of
hexane/
dichloromethane (1/2, v/v), then 11 L of
hexane/
dichloromethane (1/4, v/v). Fraction 0 containing 4 L of
hexane/
dichloromethane (2/3, v/v) was collected in four 1 L glass bottles and fractions 1-25 were collected in 500 mL glass bottles. TLC analyses of the fractions were performed on silica gel TLC plates coated with fluorescent indicator F
254 (purchased from Merck) with
hexane/
dichloromethane (1/9, v/v) as eluent and visualized with 254 nm UV light (The R
f value of
2 was 0.77). Fractions 13-24 contained the product and were combined and concentrated on a rotary evaporator (40 °C, 640 to 60 mmHg).
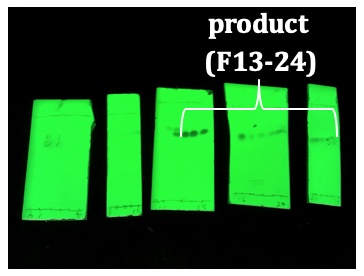
Figure 11. TLC analysis of column fractions
34. When the reaction was carried out on a 5 mmol scale, 2.62 g (64%) of the product was obtained.
35.
1,4-Bis(4-(tert-butyl)phenyl)-2,5-bis(4-cyanophenyl)-3,6-bis(4-nitrophenyl)-1,4-dihydropyrrolo[3,2-b]pyrrole (2) has the following physical properties: mp 353.0-392.8 °C (dec.); IR (film): 2962, 2869, 2226, 1599, 1515, 1341, 1115, 1587, 1017 cm
-1;
1H NMR
pdf (500 MHz, CDCl
3) δ: 1.28 (s, 18H), 6.78-6.82 (m, 8H), 7.01 (AA'XX', d,
J = 8.6 Hz, 4H), 7.11 (AA'XX', d,
J = 8.6 Hz, 4H), 7.36 (AA'XX', d,
J = 8.0 Hz, 4H), 7.75 (AA'XX', d,
J = 9.2 Hz, 4H);
13C NMR
pdf (125 MHz, CDCl
3) δ: 31.3, 34.7, 107.9, 110.6, 118.5, 122.7, 125.8, 127.3, 129.2, 130.9, 131.3, 131.8, 132.9, 135.0, 135.7, 140.1, 145.6, 151.6; HRMS (EI)
m/z calcd for C
52H
42N
6O
4Na
+ [M + Na]
+ 837.3160, found 837.3159; Anal. calcd for C
52H
42N
6O
4: C, 76.64; H, 5.19; N, 10.31. Found: C, 76.19; H, 5.43; N, 10.22. Purity was determined to be 98.3% by qNMR
pdf using 35.7 mg of
2 and 11.2 mg of
dibromomethane (Nacalai Tesque, Inc., >99.0%). The corrected weight and yield based on qNMR
pdf purity is 4.89 g (60%).
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The on-going progress in the field of organic electronics requires compounds possessing a suitable combination of photophysical and electronic properties.
2 Heteropentalenes are a class of 10Π-electron aromatic compounds. The most widely studied members of this family are thieno[3,2-
b]thiophenes and thieno[3,2-
b]pyrroles.
3 Their optoelectronic properties makes them suitable candidates in various applications.
3 Pyrrolopyrroles offer the most electron rich core among the 10Π-electron heteropentalene family.
4 In 2013 our group reported a serendipitous discovery of a multicomponent reaction between aromatic aldehydes, aromatic amines, and
butane-2,3-dione giving straightforward route to previously inaccessible 1,2,4,5-tetraarylpyrrolo[3,2-
b]pyrroles.
5 Subsequently, various catalysts have been employed and the methodology has been improved several times
6,7,8 resulting in improving the yields from ~10-20% to 50-77%. At the same time scope of this reaction has been broadened to embrace variety of primary aromatic amines and aromatic aldehydes including the derivatives of furan, benzofuran, thiophene, pyrrole, pyridine and quinoline.
7,8 Since then, we have intensively explored the chemistry of pyrrolopyrroles and their Π-extended analogs.
9 Despite their young age, tetraarylpyrrolo[3,2-
b]pyrroles have already been applied in research related to studying symmetry breaking in the excited state, organic light-emitting diodes, MOFs, solvatofluorochromism, resistive memory devices, bulk heterojunction organic solar cells, aggregation-induced emission photochromic analysis of halocarbons, and dye-sensitized solar cells.
10 Compared to thieno[3,2-
b]thiophenes, pyrrolo[3,2-
b]pyrroles offer instalment of total 6 instead of 4 substituents giving opportunity for fine tuning of the physicochemical properties. Strongly electron-rich positions 3 and 6 of pyrrolopyrroles are ideal entry points for further transformation of the central core
via electrophilic aromatic substitution, the Scholl reaction
11 or by means of direct arylation. The latter one is especially attractive given that attempts to introduce additional arene rings to positions 3 and 6 of the pyrrolopyrrole core
via traditional Suzuki-Miyaura coupling procedures turned out to be futile. After careful optimization we have discovered that the most feasible conditions for mono- and bis-arylation of tetrarylpyrrolopyrroles are the ones reported by Doucet and co-workers for the direct palladium-catalyzed polyarylation of pyrroles (Table 1).
12 Both electron-deficient and electron-rich aryl bromides proved to be good substrates under these conditions.
6 In the case of electron-donating bromoarenes, monoarylation products were almost exclusively formed (Table 1, entries 1, 7 and 8). The opposite situation emerged when haloarenes with electron-withdrawing substituents were used (Table 1, entries 2, 3, 5 and 6).
Table 1.a Palladium-catalyzed direct arylation reaction of parent tetraaryl-pyrrolo[3,2-b]pyrrole (TAPP) with various aryl bromides
Appendix
Chemical Abstracts Nomenclature (Registry Number)
4-Cyanobenzaldehyde; (105-07-7)
4-tert-Butylaniline; (769-92-6)
Fe(OTs)3· 6H2O: Iron(III) p-toluenesulfonate hexahydrate; (312619-41-3)
2,3-Butanedione; (431-03-8)
Allylpalladium(II) chloride dimer; (12012-95-2)
1-Bromo-4-nitrobenzene; (586-78-7)
DMA: N,N-Dimethylacetamide; (127-19-5)
Dppb: 1,4-Bis(diphenylphosphino)butane; (7688-25-7)

|
Maciej Krzeszewski obtained his Ph.D. from the Institute of Organic Chemistry of the Polish Academy of Sciences in 2017 under the supervision of Professor Daniel Gryko for the research on pyrrolo[3,2-b]pyrroles and their nonplanar π-extended analogs. During his Ph.D. studies, he joined Professor Valentine Vullev's group at University of California Riverside (USA) as a visiting researcher studying influence of dipole effects on electron transfer. Since 2017 to 2019, he has been working with Professor Kenichiro Itami at Nagoya University (Japan) as a JSPS postdoctoral fellow on nonplanar molecular nanocarbons. He is currently working as an assistant in Professor Daniel Gryko's group. |

|
Mariusz Tasior received his M.Sc. from the Warsaw University while conducting research under the guidance of Professor Daniel Gryko. He continued his research in the same group and obtained his Ph.D. from the Institute of Organic Chemistry of the Polish Academy of Sciences, in 2008. He worked as a postdoctoral research fellow under the supervision of Professor Donal F. O'Shea in University College Dublin (2008-2010), before obtaining a postdoctoral position at IOC PAS in Professor Gryko's group. His current research interests are focused on the synthesis and spectroscopy of advanced functional dyes with a special emphasis given to pyrrole derivatives. |

|
Marek Grzybowski obtained his Ph.D. from the Institute of Organic Chemistry of the Polish Academy of Sciences in 2014 under the supervision of Prof. D. T. Gryko. From 2015 to 2018 he carried out postdoctoral research under a JSPS fellowship in the group of Prof. S. Yamaguchi at Nagoya University aiming at the development of photostable near-infrared fluorophores for bioimaging. Currently he is an assistant professor at the Institute of Organic Chemistry PAS. His research interests are focused on the synthesis of extended π-systems and strained polycyclic arenes. |

|
Daniel T. Gryko obtained his Ph.D. from the Institute of Organic Chemistry of the Polish Academy of Sciences in 1997, under the supervision of Prof. J. Jurczak. After a post-doctoral stay with Prof. J. Lindsey at North Carolina State University, he started his independent career in Poland. He received the Society of Porphyrins and Phthalocyanines Young Investigator Award in 2008 and Foundation for Polish Science Award in 2017. His current research interests are focused on the synthesis of functional dyes as well as on two-photon absorption, excited-state intramolecular proton transfer and fluorescence probes. |

|
Shu Nakamura was born in Fukuoka, Japan. He graduated from the University of Tokyo in 2020 with B.Sc. in Pharmaceutical Sciences. He is continuing his graduate studies at the University of Tokyo under the supervision of Prof. Masayuki Inoue. His research interests are in the area of the total synthesis of complex natural products. |

|
Koichi Hagiwara was born in Kanagawa, Japan., in 1989. He received his B.Sc. degree in 2013 from the University of Tokyo, and his Ph.D. degree in Pharmaceutical Sciences in 2019 under the supervision of Prof. Masayuki Inoue. He was appointed as an assistant professor in the Graduate School of Pharmaceutical Sciences at the University of Tokyo in 2017. His research interests include the total synthesis of bioactive and highly complex natural products. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved