Org. Synth. 2021, 98, 289-314
DOI: 10.15227/orgsyn.098.0289
Synthesis of tert-Alkyl Phosphines: Preparation of Di-(1-adamantyl)phosphonium Trifluoromethanesulfonate and Tri-(1-adamantyl)phosphine
Submitted by Thomas Barber and Liam T. Ball*
1Checked by Praveen Kumar Gajula, Leila Terrab, Srinivasarao Tenneti, Gopal Sirasani, and Chris Senanayake
1. Procedure (Note 1)
A. 1-Adamantyl acetate (2). A 100 mL single-necked, round-bottomed flask equipped with a Teflon-coated stir bar (25 mm × 12 mm, oval) is charged sequentially with 1-adamantanol (15.21 g, 100 mmol, 1 equiv) (Note 2), 4-dimethylaminopyridine (1.22 g, 10.0 mmol, 0.1 equiv) (Note 3), pyridine (20 mL) (Note 4) and acetic anhydride (14.2 mL, 15.3 g, 150 mmol, 1.5 equiv) (Notes 5 and 6). The flask is fitted with an air-cooled condenser, the top of which is open to air with a 22-gauge needle (Figure 1). The flask is then lowered into an oil bath pre-heated to 60-70 °C (as measured with a thermocouple) and is stirred (500 rpm) at that temperature for 20 h (Note 7). The initial colorless suspension becomes a light-yellow solution after ca 10 min and an orange/red solution after 20 h (Figure 1A-C).
Figure 1. Step A reaction mixture after heating at 60 °C for A) 1 min, B) 10 min, C) 20 h (photo provided by checkers)
The flask is removed from the oil bath and allowed to cool in the air until the internal temperature reaches 23-27 °C, as measured with a thermometer. Stirring (500 rpm) is maintained as diethyl ether (30 mL) (Note 8) is added to the reaction flask. The resulting orange/red solution is poured into a 500 mL Erlenmeyer flask containing a Teflon-coated stir bar (40 mm × 10 mm, rod) and a saturated aqueous solution of sodium hydrogen carbonate (200 mL) (Note 9). Diethyl ether (2 × 30 mL) is used to transfer all material from the reaction flask to the Erlenmeyer flask. The biphasic mixture is stirred (300 rpm) for 5 min to control the rate of effervescence; the stirring rate is then increased to 750 rpm (5 min) and finally 1400 rpm until effervescence is no longer observed (ca 5 min). The biphasic mixture is poured into a 500 mL separatory funnel, the organic layer is separated and the aqueous layer is extracted with diethyl ether (2 × 100 mL). The combined organic layers are returned to the separatory funnel and washed with a saturated aqueous solution of copper(II) sulfate (3 × 50 mL) (Notes 10 and 11). The organic layer is transferred to a 500 mL Erlenmeyer flask and anhydrous sodium sulfate (10 g) (Note 12) is added. After swirling for 20 s, the suspension is vacuum-filtered through a fritted glass funnel (70 mm diameter sinter, medium porosity) into a 500 mL round-bottomed flask. Diethyl ether (2 × 20 mL) is used to wash the Erlenmeyer flask and filter cake. The yellow/green filtrate is concentrated by rotary evaporation (40 °C, 525-75 mmHg) to give a green liquid which solidifies on standing (Figure 2a) (Note 13). This material is transferred to a 50 mL kugelrohr distillation flask (B14 joint) using a glass Pasteur pipette (Note 14). The distillation flask is attached to a kugelrohr apparatus via two 50 mL receiver bulbs (B14 joints at both ends); the distillation flask and first receiver bulb are positioned within the kugelrohr oven, and the second receiver bulb is cooled in a dry ice/acetone bath. Rotation is started (20 rpm) and the apparatus is gradually evacuated to between 2-5×10-2 mmHg via a vacuum manifold. The crude material is distilled by heating the oven to 80 °C (30 min) and then to 85 °C (15 min) while maintaining the pressure between 2-5×10-2 mmHg. The distillate is collected in the final, cooled bulb as an off-white liquid that quickly solidifies (Figure 2b). Once distillation is complete, the apparatus is returned to atmospheric pressure, and the purified material is transferred to a pre-weighed storage vial by gentle warming with a heat gun, giving 1-adamantyl acetate 2 (17.35-17.55 g, 89.3-90.3 mmol, 89.4-90.4%, >99% purity) as a low melting solid (Figure 2b,c) (Notes 14, 15 and 16).
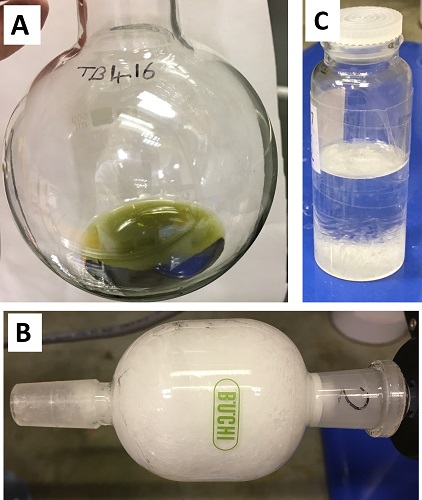
Figure 2. Step A product A) crude, B) solid in kugelrohr receiving bulb, C) after melting and transfer to vial for storage (photos provided by submitters)
B. Di-(1-adamantyl)phosphonium trifluoromethanesulfonate (3). Both chambers of a 400 mL CO-ware reactor (Notes 17 and 18) are equipped with Teflon-coated stir bars (19 mm × 10 mm, oval) (Figure 3). Chamber A is charged with zinc phosphide (78% pure, 10.0 mmol, 3.32 g, 0.5 equiv) (Note 19), and chamber B is charged with 1-adamantyl acetate 2 (11.73 g, 60.0 mmol, 3 equiv) (Notes 14 and 20). Both necks of the reactor are fitted with CO-ware PTFE-faced silicone septa (Note 21), PTFE stabilizing disks (Note 22), and screw-on lids; a 22-gauge needle (Note 23) connected via Luer lock adapter to a Schlenk line is inserted through the septum on chamber B (Note 24).
Figure 3. CO-ware reactor for step B with chambers labelled, after charging with zinc phosphide (chamber A) and 1-adamantyl acetate (chamber B) (photo provided by submitters)
The reactor is evacuated (1 x 10-2 mmHg) (Note 25) and backfilled with anhydrous dinitrogen (3 cycles). Anhydrous, degassed dichloromethane (40 mL) (Note 26) is added to chamber B by syringe (22-gauge needle), and degassed deionised water (20 mL) (Note 27) is added to chamber A by syringe (22-gauge needle). The nitrogen inlet needle is removed from chamber B, rendering the CO-ware reactor a sealed system. Both chambers of the reactor are then cooled to -78 °C (dry ice/acetone bath) without stirring, until the water in chamber A is frozen solid (Note 28). Stirring is commenced (750 rpm), then trimethylsilyl trifluoromethanesulfonate (3.62 mL, 4.44 g, 20.0 mmol, 1 equiv) (Note 29) is added to chamber B by syringe (22-gauge needle) in a single portion. The reactor is removed from the cooling bath and 10 M aqueous HCl (20 mL, 200 mmol, 10 equiv) (Notes 30 and 31) is added immediately to chamber A by syringe (22-gauge needle) in a single portion (Note 28). Stirring is maintained (750 rpm) as both chambers of the reactor are warmed in a water bath set to 50 °C (as measured by thermocouple); effervescence is observed in chamber A as the ice melts and phosphine gas (PH3, 20.0 mmol, 1 equiv) is generated (Note 32). The mixture is stirred for 4 h at 50 °C. The reactor is then removed from the heating bath and cooled to room temperature with stirring.
The gas scrubber apparatus (Figure 4) consists of three 50 mL, two-necked, round-bottomed flasks (primary neck: B24; secondary neck: B14). Flask 1 is empty, its primary neck is fitted with a rubber septum, and its secondary neck is fitted with an inlet adapter connected by PVC tubing to a Luer lock adapter fitted with a needle (22-gauge, 12 cm). The inlet adapter is secured in the secondary neck of flask 1 with a Keck clip. Flasks 2 and 3 are assembled as flask 1, but contain commercial concentrated sodium hypochlorite solution (30 mL; 10-14% w/w); their primary necks are fitted with rubber septa and the secondary neck of flask 3 is left open to air. The needle leading from flask 1 is inserted through the septum of flask 2 so that it reaches to the bottom of the sodium hypochlorite solution; the needle leading from flask 2 is inserted through the septum of flask 3 so that it reaches to the bottom of the sodium hypochlorite solution. A cannula is constructed from PVC tubing fitted with Luer lock adapters at each end; both Luer lock adapters are fitted with needles (22-gauge, 12 cm) (Figure 4b). Needle 1 of the cannula is inserted through the septum of flask A, and the scrubber system is purged with dinitrogen via needle 2 of the cannula for 10 min (Notes 34 and 35).
Needle 2 of the cannula is then inserted into chamber B of the CO-ware reactor, so that the gas released from the CO-ware reactor passes through the scrubber system. After the initial gas release has subsided (ca 10 s), a nitrogen inlet needle (Note 36) is inserted into chamber A of the CO-ware reactor and the entire system is flushed with nitrogen (Note 37) for 10 min to ensure any residual PH3 is completely purged through the bleach baths. The scrubber system is then disconnected from the CO-ware reactor.
Figure 4. Gas scrubbing system for step B. A) bleach bubbler setup with flasks labelled, B) PVC tubing cannula (photos provided by submitters)
The solution in chamber B of the CO-ware reactor is transferred by syringe (22-gauge needle) to a 250 mL round-bottomed flask, and dichloromethane (2 x 20 mL portions) is used to ensure complete transfer. The colorless solution is concentrated by rotary evaporation (40 °C, 525-75 mmHg) and the colorless phosphonium salt starts to crystallize from the remaining liquid (Note 38). A Teflon-coated stir bar (16 mm, cross) is added to the round-bottomed flask. Diethyl ether (40 mL) (Note 8) is then added in a single portion, and the mixture is stirred (500 rpm) for 15 min until the suspended solid appears completely free flowing. The crystalline solid is collected by vacuum filtration on a fritted glass funnel (30 mm diameter sinter, medium porosity). Diethyl ether (3 × 20 mL portions) is used to wash the flask and filter cake, then air is drawn through the filter cake for 20 min to give di-(1-adamantyl)phosphonium trifluoromethanesulfonate 3 (8.92 g, 19.7 mmol, 98.5%, >98% purity) as a colorless crystalline solid (Figure 5) (Notes 39, 40, and 41).

Figure 5. Step B product A) on fritted funnel, B) in vial for storage (photos supplied by submitters)
C. Tri-(1-adamantyl)phosphine (4). A 100 mL single-necked round-bottomed flask equipped with a Teflon-coated stir bar (16 mm, cross) is charged with di-(1-adamantyl)phosphonium trifluoromethanesulfonate 3 (6.79 g, 15.0 mmol, 1 equiv) and 1-adamantyl acetate 2 (3.21 g, 16.5 mmol, 1.1 equiv) (Figure 6a) (Note 14). The neck is fitted with a rubber septum and a needle (22-gauge) connected via Luer lock adapter to a dual-bank Schlenk manifold is inserted into the flask. The vessel is evacuated (2 × 10-1 mmHg) (Note 25) and backfilled with anhydrous dinitrogen (3 cycles). Anhydrous, degassed dichloromethane (40 mL) (Note 26) is added to the mixture by syringe (22-gauge needle) and stirring (500 rpm) is commenced at room temperature (Figure 6b) (Note 42). 1,8-Diazabicyclo[5.4.0]undec-7-ene (2.24 mL, 2.28 g, 15.0 mmol, 1 equiv) (Note 43) is added by syringe (22-gauge needle), affording a colorless solution (Figure 6c), which is stirred (500 rpm) at room temperature for 30 min. Trimethylsilyl trifluoromethanesulfonate (3.26 mL, 4.00 g, 18.0 mmol, 1.2 equiv) (Note 29) is added by syringe (22-gauge needle) and the resulting colorless solution (Figure 6d) is stirred (500 rpm) for 24 h at room temperature.

Figure 6. Step C reaction mixture on additions of A) 1 and 2, B) degassed, anhydrous dichloromethane, C) 1,8-diazabicyclo[5.4.0]undec-7-ene, D) trimethylsilyl trifluoromethanesulfonate (photos supplied by submitters)
Triethylamine (10.5 mL, 7.59 g, 75.0 mmol, 5 equiv) (Notes 44 and 45) is added at room temperature, and a colorless suspension forms immediately. Stirring is maintained for 30 min. The nitrogen inlet needle is then removed, the flask is opened to air and the suspended solid is immediately (Note 46) collected by vacuum filtration on a fritted glass funnel (30 mm diameter sinter, medium porosity). Ethanol (3 × 20 mL) (Notes 47 and 48) is used to wash the flask and filter cake. Air is drawn through the filter cake for 10 min, then the product is dried in vacuo (1 × 10-1 mmHg, 16 h) to give tri-(1-adamantyl)phosphine 4 (5.1 g, 11.69 mmol, 78%, >98% purity) as a colorless amorphous solid (Figure 7) (Notes 49, 50, 51, and 52).
Figure 7. Step C product A) on fritted funnel, B) in vial for storage (photos supplied by submitters)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
1-adamantanol,
4-dimethylaminopyridine,
pyridine,
acetic anhydride,
diethyl ether,
sodium hydrogen carbonate,
copper sulfate pentahydrate, concentrated
sulfuric acid,
sodium sulfate,
1,3,5-trimethoxybenzene,
chloroform-d,
zinc phosphide,
phosphine gas,
dichloromethane, deionized water,
trimethylsilyl trifluoromethanesulfonate, concentrated
hydrochloric acid,
sodium hypochlorite solution,
1,8-diazabicyclo[5.4.0]undec-7-ene,
triethylamine,
ethanol,
benzene-d6,
calcium hydride, as well as the proper procedures for performing reactions in sealed vessels under pressure and vacuum distillations. Step B involves the generation of
phosphine gas, which is pyrophoric and highly toxic; this procedure must be performed with extreme caution and only by trained scientists. Step B also involves the use of
zinc phosphide, which is highly toxic; it must be handled with care and precautions must be taken to avoid it coming into contact with acid (which will lead to the generation of
phosphine gas).
2.
1-Adamantanol (Ambeed; 97%) was used as received.
3.
4-Dimethylaminopyridine (Oakwood; 99%) was used as received..
4.
Pyridine (Sigma Aldrich; 99.8 %) was used as received.
5.
Acetic anhydride (Oakwood; 99%) was used as received.
6. The use of fewer than 1.5 equiv of
acetic anhydride results in incomplete conversion of
1-adamantanol within 20 h, and therefore reduced yields of
1-adamantyl acetate 2.
7. Reaction progress is monitored by TLC analysis on silica gel with
cyclohexane:ethyl acetate (9 : 1) as eluent. TLC plates are visualized by staining with aqueous basic
KMnO4 solution (1.5 g
KMnO4, 10 g
K2CO3 and 1.25 mL 10% wt/v NaOH in 200 mL water) followed by gentle warming with a heat gun.
Rf (
1-adamantanol) = 0.18,
Rf (
1-adamantyl acetate 2) = 0.67. (Figure 8).
Figure 8. TLC of step A crude reaction mixture after 20 h. Spots from left to right: Rxn = reaction mixture; OH = 1-adamantanol 1 (starting material), OAc = 1-adamantyl acetate 2 (authentic material from a previous batch) (photo supplied by submitters)
8.
Diethyl ether (Sigma-Aldrich; >99.8%) was used as received.
9.
Sodium hydrogen carbonate (Oakwood Chemicals; >99%) was used as received.
10. Saturated copper sulfate solution is prepared from
copper(II) sulfate pentahydrate (
ca 160 g) (Sigma-Aldrich; >98%) and deionized water (500 mL) containing 2-3 drops of conc.
sulfuric acid (Oakwood Chemicals; >98%).
11. The submitters observed that small amounts of a flocculent solid were formed during the second CuSO
4 wash. This material is removed during subsequent drying and filtration over
sodium sulfate.
12. Anhydrous
sodium sulfate (Oakwood Chemicals; >99%) was used as received.
13. Pure
1-Adamantyl acetate 2 has a melting point of 31-32 °C. Whether the crude material solidifies depends on its purity and the ambient temperature.
14. The submitters found that due to its low melting point,
1-adamantyl acetate 2 is most conveniently weighed and handled as a liquid following gentle warming with a heat gun.
15. Characterization data for
1-adamantyl acetate 2:
1H NMR
pdf (400 MHz,
CDCl3) δ: 1.60-1.71 (m, 6H), 1.96 (s, 3H), 2.08-2.12 (m, 6H), 2.12-2.19 (m, 3H);
13C NMR
pdf (101 MHz,
CDCl3) δ: 22.9, 30.9, 36.4, 41.5, 80.4, 170.5. HRMS calcd. for C
12H
18O
2+Na
+: 217.1199 [M+Na]
+; found (ESI
+): 217.1192. mp 31-32 °C. IR (ATR, neat): 2910, 2853, 1731, 1456, 1367, 1354, 1241, 1059, 1016, 864 cm
-1.
16. The purity of
1-adamantyl acetate 2 was determined to be >99% in both runs (99.9 in the example given) by quantitative
1H NMR
pdf spectroscopy in
chloroform-d with
1,3,5-trimethoxybenzene (Sigma Aldrich; 99.8%) as the internal standard (a mixture of 25.74 mg of
1-adamantyl acetate 2 and 12.45 mg of
1,3,5-trimethoxybenzene was used to determine purity of >99%).
17. The 400 mL CO-ware reactors can be purchased from Sigma Aldrich (catalog no.: STW6-1EA) or SyTracks (catalog no.: STW6).
18. Glassware used for pressurized reactions must be checked for signs of damage (including scratches, chips, and star-cracks) before use.
19.
Zinc phosphide (Sigma-Aldrich; technical grade,
ca 80% purity by weight) can be used as received, although isolated yields may be affected by using material of unknown titre. Alternatively, the purity of the
zinc phosphide can be measured by volumetric gas titration (
ACS Catal.
2020,
10, 5454-5461) before use. The submitters found that commercial,
ca 80% purity by weight
zinc phosphide (Sigma-Aldrich, technical grade) titrated to 78% purity.
20. The use of fewer than 3 equiv of
1-adamantylacetate 2 (
i.e., 1.5 equiv per P-C bond formed) leads to incomplete conversion of PH
3. This results in diminished yields and requires that the unreacted PH
3 gas must be quenched, presenting an increased safety risk.
21. PTFE-faced silicone septa for CO-ware reactors can be purchased from Sigma Aldrich (catalog no.: 743968-10EA or STW3-100EA) or SyTracks (catalog no.: STW3).
22. PTFE stabilizing disks for CO-ware reactors can be purchased from Sigma Aldrich (catalog no.: 743852-2EA or STW2-2EA) or SyTracks (catalog no.: STW2).
23. 22-Gauge needles are used to minimize damage to the CO-ware septa so that their integrity is retained once
phosphine gas is generated within the sealed reactor.
24. It is important that the needle used for evacuating and back-filling the reactor is inserted into chamber B; if the needle is inserted into chamber A, the flow of nitrogen during backfilling can blow
zinc phosphide throughout the reactor and into chamber B.
25. If the
1-adamantyl acetate 2 is a liquid at the beginning of this process, it may bubble during the first evacuation cycle, and then solidify during subsequent cycles.
26.
Dichloromethane (Sigma Aldrich; anhydrous, >99.8%) was used as received.
27. Deionized water was degassed by sparging vigorously with dinitrogen for at least 15 min before use.
28. The water in chamber A
must be frozen before addition of
hydrochloric acid. If the water is not frozen, immediate formation of
phosphine gas causes a rapid pressure increase within the apparatus; this can force the plunger from the syringe, leading to uncontrolled release of
phosphine gas.
29.
Trimethylsilyl trifluoromethanesulfonate (Oakwood Chemicals; 99%) was used as received.
30. Aqueous
hydrochloric acid (10 M) was prepared by slow addition of concentrated HCl (24.6 mL, 300 mmol; Oakwood Chemicals, 36.5-38% w/w) to deionized water (5.4 mL). The diluted acid was degassed by sparging vigorously with dinitrogen for at least 15 min before use. Note that the use of a stainless-steel needle for degassing may lead to decolorization of the acid solution, but that this does not affect the outcome of the reaction.
31. The excess
hydrochloric acid ensures complete protonolysis of the 10 mmol
zinc phosphide (
Zn3P2), which requires 60 mmol (6 equiv) of the acid to generate 20 mmol (2 equiv) of
phosphine gas.
32. Gas evolution continues for
ca 15-20 min, and is accompanied by a change in the color of the suspension in chamber A from gray to brown (Figure 9).
Figure 9. CO-ware reactor for step B, after A) 2 min and B) 20 min in the 50 °C water bath, showing suspension color change in chamber A (left hand side) (photos supplied by submitters)
33. Aqueous
sodium hypochlorite solution (Sigma-Aldrich; 10-14% w/w) was used as received.
34. To achieve nitrogen flow through the scrubber apparatus, needle 2 of the cannula is inserted through the septum of a Schlenk flask that is connected to a nitrogen manifold (
ca 1 atm overpressure). The Schlenk flask is prepared in advance by fitting a septum in the neck, then evacuating (2 × 10
-1 mmHg) and backfilling with nitrogen (3 cycles).
35. This purging stage should be used to check that the gas flow passes efficiently through the
sodium hypochlorite solution contained in flasks 2 and 3 of the scrubber system.
36. The inlet needle and tubing is purged for 10 min before use with nitrogen from a manifold at
ca 1 atm overpressure.
37. Nitrogen gas is supplied by a manifold at
ca. 1 atm overpressure.
38. In the submitters' experience, di-(1-adamantyl)phosphonium salt
3 has never failed to crystallize during rotary evaporation. However, by analogy to other di-(
tert-alkyl)phosphonium salts (
ACS Catal.
2020,
10, 5454-5461), if crystallization does not occur during rotary evaporation it should initiate upon addition of
diethyl ether and stirring vigorously.
39. Characterization data for
di-(1-adamantyl)phosphonium trifluoro-methanesulfonate 3:
1H NMR
pdf (400 MHz,
CDCl3) δ: 1.77-1.86 (br. m, 12H), 2.07-2.15 (br. m, 6H), 2.15-2.23 (br. m, 12H), 5.98 (d,
J = 469.6 Hz, 2H);
13C{
1H} NMR
pdf (101 MHz,
CDCl3) δ: 27.5 (d,
J = 10.4 Hz), 35.4 (d,
J = 34.1 Hz), 35.5 (d,
J = 2.0 Hz), 39.1 (d,
J = 2.0 Hz), 120.8 (q,
J = 321.5 Hz);
19F NMR
pdf (376 MHz,
CDCl3) δ: -78.32 (s);
31P NMR
pdf (162 MHz,
CDCl3) δ: 13.47 (t,
J = 465.6 Hz). HRMS calcd. for C
20H
32P
+: 303.2236 [M-OTf]
+; found (ESI
+): 303.2248. m.p.: >280 °C (decomposition). IR (ATR, neat): 2906, 2854, 2424, 2400, 1450, 1345, 1305, 1276, 1245, 1221, 1154, 1022, 973, 944, 898, 755 ,634, 571, 515, 489, 447, 417 cm
-1. [
Note: one of the peaks of the doublet at 35.4 ppm in the 13C{1H} NMR spectrum overlaps with the doublet at 35.5 ppm, but can be resolved with additional spectral processing].
40. A reaction performed on half-scale provided 4.46 g (99%) of the product.
41. The purity of
di-(1-adamantyl)phosphonium trifluoromethanesulfonate 3 prepared in this way was determined to be >98% from both runs (98.9% in the example given) by quantitative
1H NMR
pdf spectroscopy in
chloroform-d with
1,3,5-trimethoxybenzene (Sigma Aldrich; 99.8%) as the internal standard (a mixture of 5.65 mg of
di(1-adamanty)phosphonium trifluoromethanesulfonate 3 and 4.15 mg of
1,3,5-trimethoxybenzene was used to determine purity).
42. The reaction mixture is a colorless suspension containing a small amount of undissolved solid material.
43.
1,8-Diazabicyclo[5.4.0]undec-7-ene (Sigma Aldrich; 99%) was dried by stirring over
calcium hydride (Sigma Aldrich) for 4 h, then distilled from
calcium hydride in flame-dried apparatus at 1.5-2 × 10
-1 mmHg. The distilled reagent was stored under anhydrous dinitrogen in a flame-dried vessel sealed with a J-Young's tap closure.
44.
Triethylamine (Sigma Aldrich; ≥99%) was dried by stirring over
calcium hydride (Sigma Aldrich) for 4 h, then distilled from
calcium hydride in flame-dried apparatus under anhydrous dinitrogen. The distilled reagent was stored under anhydrous dinitrogen in a flame-dried vessel sealed with a J-Young's tap closure.
45. The use of excess
triethylamine drives the equilibrium deprotonation of the initially-formed tri-(1-adamantyl)phosphonium triflate salt, and hence precipitation of
tri-(1-adamantyl)phosphine 4.
46. The
dichloromethane supernatant begins to discolor to yellow (Figure 10) immediately on exposure to air, so it is important to begin the filtration and washing procedure quickly.
Figure 10. Discoloration of dichloromethane supernatant (photo is of collected dichloromethane/ethanol filtrate) (photo supplied by submitters)
47.
Ethanol (Sigma Aldrich; >99.8%) was used as received.
48. Although the submitters found that washing with three portions of
ethanol was sufficient to completely remove the yellow color from the product, additional washes may be used as required.
49.
Tri-(1-adamantyl)phosphine 4 is air stable in the solid state, but oxidizes in solution and reacts with chloroform. Samples for NMR spectroscopic analysis must therefore be prepared and sealed under an inert atmosphere using an appropriate solvent (
e.g.,
benzene-d6). The
benzene-d6 solvent (Sigma-Aldrich; 99.6 %D) was used as received. The NMR sample was prepared in a glove box under inert atmosphere.
50. Characterization data for
tri-(1-adamantyl)phosphine 4:
1H NMR
pdf (400 MHz,
C6D6) δ: 1.63-1.71 (m, 9H), 1.73-1.80 (m, 9H), 1.93 (brs, 9H), 2.37 (brs, 18H);
13C NMR
pdf (101 MHz,
C6D6) δ: 29.8 (d,
J = 7.1 Hz), 37.4, 41.6 (d,
J = 36.6 Hz), 43.2 (br);
31P NMR
pdf (162 MHz,
C6D6) δ: 59.22. HRMS calcd. for C
30H
46P
+: 437.3332 [M+H]
+; found (ESI
+): 437.3343. mp >280 °C (decomposition). IR (ATR, neat): 2900, 2874, 2844, 1449, 1341, 1297, 1254, 1180, 1101, 1037, 966, 937, 928, 877, 817, 645, 482, 415 cm
-1.
51. Reactions performed on half-scale provided 2.5 g (76%) of the product.
52. The purity of
tri-(1-adamantyl)phosphine 4 was determined to be 98% from both runs (98.1 in the example shown) by quantitative
1H NMR
pdf spectroscopy in benzene-
d6 (
Note 48) with
1,3,5-trimethoxybenzene (Sigma Aldrich; 99.8%) as the internal standard (a mixture of 4.5 mg of
tri-(1-adamantyl)phosphine 4 and 4.0 mg of
1,3,5-trimethoxybenzene was used to determine purity).
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
phosphine ligands play an indispensable role in homogeneous catalysis, providing chemists with the ability to finely tune catalyst activity, stability and selectivity. Among the many extant phosphine classes, those bearing tertiary alkyl substituents have emerged as especially privileged in fields as diverse as polymerization,
2 strong-bond activation
3 and cross-coupling.
4
Despite their privileged status, the synthesis of
tert-alkyl phosphines remains challenging. The vast majority of syntheses depend on step-wise addition of
tert-alkyllithium or Grignard reagents to PCl
3 (Scheme 1A); primary and secondary phosphines are then obtained by reduction of the corresponding
P-chloro phosphines with LiAlH
4, whereas tertiary phosphines are accessed by completing the third alkylation with excess nucleophile and a (catalytic) metal additive.
5 While the synthesis of di(adamantanoid) phosphines notably avoids the use of organometallic reagents, it still employs highly reactive air and moisture sensitive reagents (Scheme 1B).
6Scheme 1. tert-Alkyl phosphines are conventionally synthesized over multiple steps from PCl3, using highly reactive reagents
The need to handle highly reactive reagents agents, use toxic PCl3, and manipulate, purify and isolate air-sensitive intermediates over multiple steps renders the de novo synthesis of tert-alkyl substituted phosphines non-trivial. Combined with the lack of commercially available organometallic reagents or adamantanes, these practical difficulties have limited the diversity of the tert-alkyl substituents that can be installed at phosphorus.
We recently developed a general and practical method for the synthesis of di-
tert-alkyl phosphine building blocks
via the S
N1 alkylation of phosphine gas (Scheme 2A).
7 This umpolung strategy uses esters as benign, bench-stable alkylating agents which are readily prepared from commercially available tertiary alcohols. Although
phosphine gas is pyrophoric and toxic, it is used as the limiting reagent and is prepared on demand within a sealed system by protonolysis of
zinc phosphide, a cheap, air and water-stable solid. The di-
tert-alkyl phosphine product is formed as the corresponding air-stable phosphonium salt, which is isolated from the reaction mixture in high purity by filtration under air.
Scheme 2. Synthesis of air-stable, odorless di-tert-alkylphosphonium salts via SN1 alkylation of ex situ generated phosphine gas. Yields are of isolated, purified materials
Our SN1 alkylation methodology can be applied successfully to a range of diverse tert-alkyl esters, thereby providing facile access to novel di-tert-alkyl phosphonium building blocks (Scheme 2B). These salts can be used conveniently in ligand synthesis: treatment with base releases the corresponding phosphine in situ, prior to P-functionalization via established methods (Scheme 3).
Scheme 3. Use of di-tert-alkylphosphonium salts in common P-functionalization reactions. Yields are of isolated, purified materials
One tri-
tert-alkyl phosphine that has garnered much recent attention is
tri(1-adamantyl)phosphine 4.
2c,2d,8 This bulky, electron rich
phosphine had eluded synthesis by conventional methods until 2016, when Carrow demonstrated that it could be accessed efficiently by S
N1 alkylation of commercial di(1-adamantyl)phosphine.
9 We recognized that we could adapt Carrow's pioneering procedure to start from our stable di(1-adamantyl)phosphonium salt
3; in this way we would provide a practical route to substantial amounts of a best-in-class ligand that avoids using an expensive, air-sensitive secondary
phosphine.
To showcase the scalability and reproducibility of our SN1 alkylation methodology, and the versatility of its products in synthesis, we therefore chose to make di(1-adamantyl)phosphonium triflate 3 and subsequently tri(1-adamantyl)phosphine 4.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
1-Adamantanol: tricyclo[3.3.1.13,7]decan-1-ol; (768-95-6)
Acetic anhydride: acetic acid, anhydride; (108-24-7)
Triethylamine: ethanamine, N,N-diethyl-; (121-44-8)
4-Dimethylaminopyridine: 4-pyridinamine, N,N-dimethyl- (1122-58-3)
Pyridine: pyridine; (110-86-1)
Cyclohexane: cyclohexane; (110-82-7)
KMnO4: permanganic acid, (HMnO4), potassium salt; (7722-64-7)
K2CO3: carbonic acid, dipotassium salt; (584-08-7)
Sodium hydrogen carbonate: carbonic acid monosodium salt; (144-55-8)
Copper(II) sulfate pentahydrate, CuSO4: sulfuric acid copper(2+) salt (1:1), pentahydrate; (7758-99-8)
Sulfuric acid: sulfuric acid; (7664-93-9)
Magnesium sulfate: sulfuric acid magnesium salt (1:1); (7487-88-9)
Zinc phosphide: zinc phosphide, (Zn3P2); (1314-84-7)
Trimethylsilyl trifluoromethanesulfonate: methanesulfonic acid, trifluoro-, trimethylsilyl ester; (27607-77-8)
Hydrochloric acid, HCl: hydrochloric acid; (7647-01-0)
Sodium hypochlorite: hypochlorous acid, sodium salt; (7681-52-9)
1,8-Diazabicyclo[5.4.0]undec-7-ene: pyrimido[1,2-a]azepine, 2,3,4,6,7,8,9,10-octahydro-; (6674-22-2)
Calcium hydride: calcium hydride, (CaH2); (7789-78-8)
1,3,5-Trimethoxybenzene: (621-23-8)
Chloroform-d, CDCl3: methane-d, trichloro-; (865-49-6)
Benzene-d6, C6D6: benzene-d6; (1076-43-3)
Tri-(1-adamantyl)phosphine: (4) (897665-73-5)

|
Tom Barber obtained his MChem from Durham University, UK, following research with Dr Chris Coxon and Prof. Andy Whiting. Since 2016, he has been working towards his Ph.D. in organophosphorus chemistry and catalysis with Dr Liam Ball at the University of Nottingham. |

|
Liam Ball obtained his MSc from the University of Bristol, UK. Following doctoral studies with Dr. Chris Russell and Prof. Guy Lloyd-Jones FRS FRSE at the University of Bristol (2009-2013), he moved to the University of Edinburgh, UK, as a postdoctoral researcher with Prof. Guy Lloyd-Jones FRS FRSE (2014-2015). In 2015, Liam was appointed Assistant Professor of Organic Chemistry at the University of Nottingham. |

|
Praveen Kumar Gajula received his Bachelor's and Master's degrees from Hyderabad. He obtained his Ph.D. in synthetic organic chemistry in 2012 from CSIR-IICT, Hyderabad under the guidance of Dr. Tushar Kanti Chakraborty. His research was focused on amide-modified RNA mimics, total syntheses of natural products, anti-cancer compounds and their analogs thereof. In 2019, he joined Prof. Eriks Rozners research group at Binghamton University, optimization of RNA interference (RNAi) and CRISPR efficiency and specificity using chemically modified RNAs. In 2015, Praveen joined Sai Life Sciences, Hyderabad, India. He is currently working at TCG GreenChem, Inc. as a Senior Research Scientist in the department of process research and development. |

|
Leila Terrab obtained her Ph.D. in synthetic organic chemistry in 2021 from the University of Pittsburgh under the guidance of Dr. Peter Wipf. Her doctoral studies focused on the synthesis of small molecule inhibitors of the Artemis endonuclease, as well as the synthesis of HSP70 agonists. In 2021, Leila joined TCG GreenChem, Inc. as a postdoctoral scientist in Process Chemistry. |

|
Srinivasarao Tenneti received his Ph.D. in Chemistry under Dr. J. S. Yadav at Indian Institute of Chemical Technology, India in 2013. He worked with Professor Philip S. Low at Purdue University as a Postdoctoral Fellow, and in 2016, he joined Professor T. V. RajanBabu at The Ohio State University as a Postdoctoral Researcher, where he worked on synthesis of natural products using nickel-catalyzed asymmetric hydrovinylation methodology. He then moved to Univ. of Florida in 2019 as a Postdoctoral Associate under the supervision of Professor Robert W. Huigens III where his research was focused on synthesis of indole alkaloids. Currently, he is working as a senior scientist at TCG GreenChem, Inc. |

|
Dr. Gopal Sirasani received his Bachelor's and Master's degrees in Hyderabad, India. He obtained his Ph.D. in synthetic organic chemistry in 2011 from Temple University, Philadelphia under the guidance of Prof. Rodrigo B. Andrade. His doctoral research was focused on developing novel methodologies, total syntheses of natural products and their analogs thereof. He got his post-doctoral training in the laboratory of Prof. Emily Balskus at Harvard University, where he developed biocompatible organic reactions utilizing microbially-generated reagents to realize transition metal catalysis in the presence of microbes. In 2013, Gopal began his industrial career at Melinta Therapeutics, New Haven, CT. He is currently working at TCG GreenChem, Inc. as a Director in the department of process research and development. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved