Org. Synth. 2021, 98, 363-373
DOI: 10.15227/orgsyn.098.0363
C2 Amination of Pyridine with Primary Amines Mediated by Sodium Hydride in the Presence of Lithium Iodide
Submitted by Jia Hao Pang, Derek Yiren Ong, and Shunsuke Chiba*
1Checked by Simon Cooper and Sarah Reisman
1. Procedure (Note 1)
N-Butylpyridin-2-amine (3). An oven-dried, 250 mL three-necked round-bottomed flask fitted with a 25 x 15 mm Teflon-coated oval magnetic stir bar, a thermometer, rubber septum and a Liebig reflux condenser, is connected to a Schlenk line (Note 2). Sodium hydride (NaH) (3.00 g, 75.0 mmol, 3.0 equiv) (Note 3) is charged to the reaction vessel, after which it is evacuated and backfilled with argon three times. Anhydrous THF (Note 4) (35 mL) is added via a syringe. The reaction flask is suspended in a 22 °C water bath, and lithium iodide (LiI) (6.69 g, 50.0 mmol, 2.0 equiv) (Note 5) is introduced through the top of the reflux condenser, after which the Ar line is reintroduced with an out needle in place for 5 min to exchange the atmosphere. The addition of LiI to the reaction mixture resulted in an exotherm which causes the internal temperature to rise to 35 °C. The reaction mixture is allowed to cool to 25 ℃ under Ar atmosphere while stirring (Note 6), at which time n-butylamine (2) (5.00 mL, 50.6 mmol, 2.0 equiv) (Note 7) is added to the grey suspension in one portion using a syringe. Pyridine (1) (1.985 g, 25.1 mmol, 1.0 equiv) (Note 8) is weighed into a dry 4 mL vial and added via syringe in one portion. Tetrahydrofuran (THF) (15 mL) is used to rinse the vial and ensure complete transfer of the pyridine. (Figure 1). The grey suspension is heated at reflux temperature in a silicone oil bath for 24 h (Note 9), resulting in gradual formation of a greyish-yellow suspension (Figures 2 and 3) (Note 10).
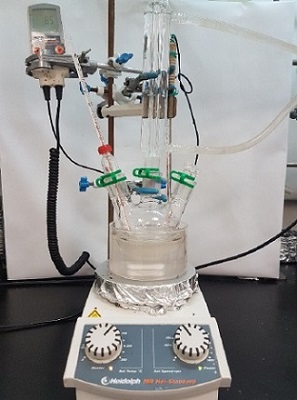
Figure 1. Reaction setup (photo provided by submitters)
Figure 2. Color change observed from a grey suspension to a greyish-yellow suspension (photo provided by submitters)
Figure 3. TLC visualization of the reaction mixture
(photo provided by submitters)
The reaction mixture is cooled to 10 ℃ (internal temperature) with an ice water bath. Cold water (15 mL) is added dropwise over 5 min before further addition of cold water (85 mL) in one portion. The layers are partitioned in a 500-mL separatory funnel. The aqueous layer is extracted with ethyl acetate (150 mL x 3), which is combined, dried over anhydrous sodium sulphate (55 g), and filtered with a Büchner funnel fitted with a fritted disk (medium porosity). The filtrate is concentrated on a rotary evaporator under reduced pressure (40 ℃, 20 mmHg) to afford an orange solid (Figure 4).
Figure 4. Orange solids obtained after workup
(photo provided by submitters)
The crude compound is stored in a freezer at -20 °C until purification by column chromatography (Note 11) to afford N-butylpyridin-2-amine (3) (Notes 12, 13, and 14) as pale-yellow solids (3.42 g, 22.8 mmol, 91%) (Figure 5) (Note 15).
Figure 5. Pale-yellow solids of N-butylpyridin-2-amine (3)
(photo provided by submitters)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regards to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
sodium hydride,
lithium iodide,
pyridine,
n-butylamine,
hydrogen gas,
sodium sulfate,
THF,
hexane and
ethyl acetate.
2. The glass joints were greased thoroughly to minimize solvent evaporation over the course of the reaction.
3.
Sodium hydride (60% dispersion in mineral oil) was purchased from Sigma Aldrich and used without any further purification. Submitter stored the reagent in an argon-filled glovebox.
4.
Tetrahydrofuran (HiPerSolv CHROMANORM® for HPLC) was purchased from VWR Chemicals and was further purified using Pure Solv MD-5 solvent purification system by Innovative Technology before use.
5.
Lithium iodide (anhydrous, beads, -10 mesh, 99.99% trace metal basis) was purchased from Sigma-Aldrich. The reagent was added to the reaction mixture immediately following opening of the ampule containing it.
6. The reaction was kept stirring at 500 rpm after the addition of
tetrahydrofuran.
7.
n-Butylamine (
2) (99.5%) was purchased from Sigma Aldrich and used without any further purification.
8.
Pyridine (
1) (anhydrous, 99.8%) was purchased from Sigma Aldrich and used without any further purification.
9. The thermocouple was set to 85 °C to ensure a strong reflux. The oil bath temperature fluctuated between ±5 °C. The internal temperature of the reaction mixture was consistently at 66 °C.
10. The reaction was monitored by TLC (EMD Millipore™ TLC Silica Gel 60 F254, glass plates) on silica gel using hexanes/
EtOAc (80:20) as eluent and visualization with UV light (Spectroline Longlife Filter Ultraviolet Light, ENF-240C, shortwave UV 254 nm). Product
3 had R
f = 0.42 and
pyridine (
1) had R
f = 0.22.
11. Column chromatography was performed using a Synthware chromatography column with a 500 mL reservoir: ID x Length (40.0 x 203 mm). The column was packed with 90 g of silica gel, high-purity grade, pore size 60 Å, 230-400 mesh particle size purchased from Sigma-Aldrich and conditioned with 500 mL hexanes. The crude mixture was mixed with silica (10 g) and
ethyl acetate (10 mL), evaporated to dryness (40 °C, 20 mmHg), and dry-loaded onto the column. The column was eluted with hexanes/
EtOAc (0.3 L, 95:5), followed by hexanes/
EtOAc (1 L, 90:10) and hexanes/
EtOAc (2 L, 80:20). Fractions sizes of 50 mL were collected. The fractions containing product show a UV-active/KMnO
4-active spot at R
f = 0.4 in hexanes/
EtOAc (80:20). The compound was concentrated on a rotary evaporator under reduced pressure (40 °C, 20 mmHg). The compound was placed under vacuum (0.75 mmHg) until it solidifies after which it was rinsed with hexanes (10 mL) and concentrated again on the rotary evaporator under reduced pressure (40 °C, 20 mmHg) to remove any residual solvents. The compound was further dried overnight under vacuum (room temperature, 0.75 mmHg).
12.
N-Butylpyridin-2-amine (
3) is bench stable. It displays the following characterization data: mp 38-39 °C; R
f = 0.42 (hexanes/
EtOAc 80:20);
1H NMR
pdf (400 MHz, CDCl
3) δ: 0.95 (t,
J = 7.3 Hz, 3H), 1.33 - 1.51 (m, 3H), 1.51 - 1.68 (m, 2H), 3.18 - 3.31 (m, 2H), 4.48 (s, 1H), 6.36 (dt,
J = 8.4, 0.9 Hz, 1H), 6.54 (ddt,
J = 6.9, 5.1, 0.9 Hz, 1H), 7.35 - 7.46 (m, 1H), 8.07 (ddd,
J = 5.0, 1.9, 0.9 Hz, 1H);
13C NMR
pdf (101 MHz, CDCl
3) δ: 13.9, 20.2, 31.7, 42.0, 106.3, 112.6, 137.4, 148.2, 159.0; FTIR (neat) 3252, 3094, 3013, 2955, 2924, 2959, 1607, 1574, 1531, 1451, 1435, 1393, 1331, 1290, 1271, 1240, 1153, 1142, 1084, 980, 906, 840, 768, 748, 735, 637, 619, 600, 517 cm
-1.
13. Purity was determined to be 98.0% on the first run using qNMR
pdf (relaxation delay of 60 s) with 1,3,5-trimethoxybenzene as an internal standard.
14. Based upon the purity of 98%, the amount of material produced was 3.35 g (89%).
15. A second run by the checkers on a scale of 25.1 mmol provided 3.35 g (89%) of the same product with similar purity.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
It is known that the reaction of
pyridine (
1) with sodium amide (NaNH
2) gives 2-aminopyridine, which is known as the Chichibabin reaction (amination).
2,3 However, variants of the Chichibabin amination that engage primary alkyl amines as a nucleophile are scarce.
4Our group recently reported a concise protocol on the Chichibabin amination of
pyridine (
1) with primary alkyl amines using
sodium hydride (
NaH) in the presence of
lithium iodide (
LiI) in
THF.
5,6 The optimization of the reaction conditions (Table 1) revealed that the reaction of
pyridine (
1) and
n-butylamine (
2) (2 equiv) could be facilitated in the presence of
NaH (3 equiv) and
LiI (2 equiv) in
THF under reflux conditions (at 66 °C). The reaction could be completed within 18 h in 0.5 mmol scale of
1 (entry 1) and 24 h in 25 mmol scale of
1 (entry 2, see the detailed protocol above). Although higher reaction temperature (85 °C) in sealed conditions (in 0.5 mmol scale of
1) allowed for completion of the process within 7 h (entry 3), the sealed reaction setting is not advisable, especially in the larger scale, since the process produces 2 molar equivalents of
hydrogen (
H2) gas for each aminated product that is formed.
Table 1. Optimization of the reaction conditions
The protocol could engage various sets of primary alkyl amines (Scheme 1). It should be noted that the present protocol is thus far proven unsuccessful for use of anilines and their derivatives.
Scheme 1. Examples of C2-aminated pyridines synthesized by the present protocol
The present protocol allows for two-fold amination of 2,2'-bipyridine in good yield (Scheme 2).
Scheme 2. Two-fold amination of 2,2'-bipyridine
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Sodium hydride; (7646-69-7)
Lithium iodide; (10377-51-2)
Tetrahydrofuran; (109-99-9)
Pyridine; (1) (110-86-1)
n-Butylamine; (2) (109-73-9)

|
Jia Hao Pang completed his undergraduate studies at Nanyang Technological University (NTU) Singapore in 2016 before beginning his PhD work in the laboratory of Shunsuke Chiba at NTU. He is currently focusing on chemistry of main group metal hydrides for methodology development. |

|
Derek Yiren Ong completed his undergraduate studies at Nanyang Technological University (NTU) Singapore in 2013. After working as an NMR technician at NTU for several years, he started his Ph.D. work in the laboratory of Shunsuke Chiba and earned his Ph.D. in 2020. He is currently working at JEOL Asia Pte. Ltd. as an application specialist in NMR. |

|
Shunsuke Chiba earned his Ph.D. in March 2006 from the University of Tokyo under the supervision of Prof. Koichi Narasaka. In 2007, he embarked on his independent career as the faculty of Nanyang Technological University (NTU) Singapore, where he is currently Professor of Chemistry. His research group focuses on methodology development in the area of synthetic chemistry and catalysis. |

|
Simon Cooper completed his undergraduate studies at the University of San Francisco in 2015. He then joined Prof. Todd Hyster's laboratory developing biocatalytic methods at Princeton University and completed his PhD in 2020. He is currently working on natural product synthesis as a postdoctoral fellow with Prof. Sarah Reisman. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved