Org. Synth. 2021, 98, 391-406
DOI: 10.15227/orgsyn.098.0391
Preparation of (Bis)Cationic Nitrogen-Ligated I(III) Reagents: Synthesis of [(pyridine)2IPh](OTf)2 and [(4-CF3-pyridine)2IPh](OTf)2
Submitted by Bilal Hoblos and Sarah E. Wengryniuk
1*
Checked by Jeffrey T. Kuethe and Kevin R. Campos
1. Procedure (Note 1)
A. [(Pyridine)2IPh](OTf)2 (2). A 100 mL three-necked (24/40) round-bottomed flask containing an egg-shaped Teflon-coated stir bar (12.5 mm x 25 mm) (Note 2) is evacuated (Note 3) and flame-dried (Note 4). After cooling to room temperature, the flask is backfilled with argon and charged with diacetoxyiodobenzene (PhI(OAc)2); (2.00 g, 6.21 mmol, 1 equiv) (Note 5), and the three necks are equipped with rubber septa. A positive pressure of argon is provided through a needle inserted into one of the septa. Diethyl ether (62 mL) (Note 6) is then added via syringe to generate a white, cloudy solution (Figure 1a). Stirring is initiated (Note 7), and, at room temperature, trimethylsilyl trifluoromethanesulfonate (2.25 mL, 12.4 mmol, 2 equiv) (Note 8) is then added via syringe over a period of 10 sec.
Figure 1. Appearance of reaction while stirring (a) before addition of TMSOTf, (b) immediately after full addition of TMSOTf
(photos provided by checkers when using a single-necked flask)
The resulting mauve-colored, slightly cloudy mixture is stirred for 10 minutes, over which time the solution becomes homogenous and more yellow in appearance (Figure 1b). Pyridine (1.0 mL, 12.4 mmol, 2 equiv) (Note 9) is added via syringe over a period of 20 sec. Immediately upon addition of pyridine, the desired product precipitates as an off-white solid (Figure 2a). Stirring is continued for 15 min, which is the time required for any precipitate or residue stuck to the side of the flask to be liberated (Note 10). The septum on the left most neck of the reaction flask is replaced with a 24/40 glass vacuum adapter connected to a Schlenk manifold flowing a positive pressure of argon.
A second 100-mL three-necked (24/40), round-bottomed flask equipped with two glass stoppers and a glass vacuum adapter is evacuated (Note 3) and flame-dried (Note 4). A positive pressure of argon is provided via the adapter, and the flask is allowed to cool to room temperature. The stopper on the leftmost neck of the empty three-necked flask is removed and replaced by a dry double male joint filter stick (Note 11). The septum on the central neck of the reaction flask is then replaced with the other, shallow end of the filter stick (Figure 2b). The ground glass joints are wrapped with parafilm and/or secured with Keck clamps. The entire apparatus is then flipped, and the suspension is allowed to fall into the filterstick. The receiving flask is submerged in a -78 ºC dry ice/acetone bath to limit filtrate evaporation. Argon flow to the reaction flask is increased and a vacuum is applied to the receiving flask via the corresponding vacuum adapters connected to the Schlenk manifold. After the solvent has fully passed through the material into the receiving flask, the vacuum line is closed, and the three-necked reaction flask is removed from the top of the filter stick to allow for the addition of rinsing solvent (diethyl ether (Note 6) and agitation of the product cake. Between rinses, a dry one-necked flask is placed on the top of the filter stick (Figure 2b). The flask is then replaced with a female 24/40 glass vacuum adapter flowing a positive pressure of argon, and the vacuum line on the receiving flask is then opened, to allow solvent to pass through the filter. This process is performed twice with diethyl ether (2 x 25 mL), and the product is allowed to dry on the filter for an additional 2 min.

Figure 2. (a) Reaction mixture immediately after addition of pyridine (illustrated using a one-necked flask), and (b) reaction product during filtration
(photos provided by checkers)
The product is quickly transferred to a pre-weighed, flame-dried, single-necked (24/40) 100 mL round-bottomed flask for drying. The flask is evacuated and left under constant vacuum for 1 h (Note 3), then backfilled with argon to reveal the product as a free-flowing, off-white powder (3.81 g, 91%) (Notes 12 and 13). The product is transferred to a flame-dried product vial (20 mL) for long term storage in a desiccator (Note 14).
B. [(4-(Trifluoromethyl)pyridine)2IPh](OTf)2 (3). A 100-mL three-necked (24/40) round-bottomed flask containing an egg-shaped Teflon-coated stir bar (12.5mm x 25mm) (Note 2) is evacuated (Note 3) and flame-dried (Note 4). After cooling to room temperature, the flask is backfilled with argon and charged with diacetoxyiodobenzene (PhI(OAc)2; 2.00 g, 6.21 mmol, 1 equiv) (Note 5), and the three necks are equipped with rubber septa, one of which is pierced with a needle that is delivering a positive pressure of argon. Diethyl ether (62 mL) (Note 6) is then added via syringe to generate a white, cloudy solution. Stirring is initiated (Note 7), then, at room temperature, trimethylsilyl trifluoromethanesulfonate (2.25 mL, 12.4 mmol, 2 equiv) (Note 8) is added via syringe over a period of 10 sec.
The resulting mauve colored, slightly cloudy mixture is stirred for 10 min, over which time the solution becomes homogeneous and yellow in appearance. 4-(Trifluoromethyl)pyridine (1.44 mL, 12.4 mmol, 2 equiv) (Note 9) is added by syringe over a period of 20 sec. Immediately upon addition of the heterocycle, the off-white product precipitates (Figure 3). Stirring is continued for 15 min, which is the time required for any precipitate or residue stuck to the side of the flask to be liberated (Note 10). The septum on the leftmost neck of the reaction flask is replaced with a 24/40 glass vacuum adapter connected to a Schlenk manifold flowing a positive pressure of argon.
To effect isolation of the product (Note 15) a second 100-mL three-necked (24/40), round-bottomed flask is equipped with two glass stoppers and a vacuum adapter. The flask is evacuated (Note 3) and flame-dried (Note 4). A positive pressure of argon is provided via the adapter, and the flask is allowed to cool to room temperature. The stopper on the leftmost neck of the empty three-necked flask is removed and replaced by the shallow end of a dry double male end adapter filter stick (Note 11). The septum on the central neck of the reaction flask is then replaced with the other end of the filter stick (Figure 2b). The ground glass joints are wrapped with parafilm and/or secured with Keck clamps. The entire apparatus is then flipped, and the suspension is allowed to fall into the filter stick, and the receiving flask is submerged in a -78º C dry ice/acetone bath to limit filtrate evaporation. Argon flow to the reaction flask is increased and a vacuum is applied to the receiving flask via the corresponding vacuum adapters connected to the Schlenk line. After the solvent has fully passed through the material into the receiving flask, the vacuum line is closed, and the three-necked reaction flask is removed from the top of the filter stick to allow for the addition of rinsing solvent (diethyl ether (Note 6)) and agitation of the product cake. Between rinses, a dry one-necked flask is placed on the top of the filter stick (Figure 2b). The flask is then replaced with a female 24/40 glass vacuum adapter flowing a positive pressure of argon from the Schlenk manifold, and the vacuum line on the receiving flask is then opened, to allow solvent to pass through the filter. This process is performed twice with diethyl ether (2 x 25 mL), and the product is allowed to dry on the filter for an additional 2 min.
The product is quickly transferred to a pre-weighed, flame-dried, single-necked (24/40) 100 mL round-bottomed flask for drying. The flask is evacuated and left under constant vacuum for one h (Note 3), then backfilled with argon to reveal the product as a free-flowing, off-white powder (4.40 g, 89%) (Notes
16 and
17). The product is transferred to a flame-dried product vial (20 mL) for long term storage in a desiccator (
Note 15).
Figure 3. Reaction mixture immediately after addition of 4-CF3-pyridine
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with (
diacetoxyiodobenzene (
PhI(OAc)2),
diethyl ether,
trimethylsilyl trifluoromethanesulfonate,
pyridine,
4-(trifluoromethyl)pyridine,
dichloromethane,
calcium hydride,
calcium sulfate,
acetone, dry ice, and
methanol.
2. The shape of the stir bar is important as a thick slurry forms after the addition of the heterocycle, and the slurry can stick to the inner walls of the flask. The shape of the stir bar allows for greater surface contact with the flask and more uniform stirring of the slurry.
3. An Edwards RV12 direct-drive pump was used to create the vacuum (2-6 mmHg) during the evacuation of the glassware, as well as for the removal of residual solvent from products.
4. The checkers dried the reaction flask in an oven heated at 120 ℃ for 24 h prior to use and cooled the flask under an atmosphere of
nitrogen. The submitters flame-dried the glassware. The flask (under vacuum via a needle through a rubber septum) is subjected to a torch flame from a bernzomatic-brand butane torch for 20 sec, ensuring all surfaces of the glass receive nearly equal heat. The flask is allowed to cool under vacuum (
Note 3).
5.
Diacetoxyiodobenzene,
PhI(OAc)2, was purchased by the submitters from Oakwood Chemical (98% purity) and used without further purification. The checkers purchased
PhI(OAc)2 from Chem-Impex Int'L, Inc. (99.4%, HPLC).
6. The checkers purchased anhydrous
diethyl ether from Sigma Aldrich in Sure/Seal
TM bottles which were used as received. The submitter's solvents, including
diethyl ether, were purchased from Fisher Scientific (HPLC grade passed through an activated alumina column).
Diethyl ether is the preferred solvent for the examples provided herein. However, if the heterocycle used is found to be poorly soluble in
diethyl ether,
dichloromethane should be substituted since low yields and byproduct formation are observed when the heterocycle is not completely solubilized.
7. The stir plate used was a Corning PC351 and stir rate was around 600 rpm throughout the experiment.
8.
Trimethylsilyl trifluoromethanesulfonate (
TMSOTf) was purchased by the submitters from Oakwood Chemical (99% reagent grade). The reagent was distilled over
calcium hydride and stored over activated 3Å molecular sieves (Sigma Aldrich). The checkers purchased
TMSOTf from Sigma Aldrich in sealed ampules and were used as received.
9.
Pyridine was purchased by the submitters from Fisher Scientific (>99% ACS grade), distilled over
calcium hydride, and stored over activated 3Å molecular sieves. The checkers purchased anhydrous
pyridine from Sigma Aldrich in Sure/Seal
TM bottles which were used as received. Both submitters and checkers purchased 4-CF
3-pyridine (99%) from Oakwood. The material was purified and stored in the same fashion as described above for
pyridine.
10. In some cases, the stir bar was unable to reach the solids that were stuck to the walls of the flask. In this case, the flask was removed from the clamp and manually agitated (swirled and lightly shaken) until the walls of the flask were sufficiently cleared of all residue.
11. Complex glassware was dried by placement in an oven (150 °C) and was cooled in a glass desiccator filled with
calcium sulfate (Drierite) dessicant.
12. Characterization data for
Py-HVI (
2):
1H NMR
pdf (500 MHz, CD
3OD) δ: 7.67 (t, 2H,
J = 7.7 Hz), 7.80 (t, 1H,
J = 7.4 Hz), 7.95 (t, 4H,
J = 5.7 Hz), 8.31 (d, 2H,
J = 8.0 Hz), 8.48 (t, 2H,
J = 7.8 Hz), 8.79 (d, 4H,
J = 5.7 Hz);
13C NMR
pdf (125 MHz, CD
3OD) δ: 121.7 (q,
J = 396.2 Hz), 122.4, 128.2, 132.8, 134.5, 136.4, 144.5; 146.0;.
19F NMR
pdf (470 MHz, CD
3OD) δ: -80.08; IR (ATR): 3084, 2360, 1605, 1242, 1155, 1025, 753, 681, 630. Elemental Analysis: calc'd; C: 32.74, H: 2.29, N: 4.24, Found; C: 31.95, H: 2.38, N: 4.06 (submitter's data) Due to instability and rapid decomposition, the checkers were unable to obtain purity data. Due to inherent instability, melting point was not determined.
13. Two additional reactions checked on the same scale provided 3.82 g (93%) and 3.81 g (93%), respectively.
14. The product should be stored in a cool, dry desiccator to avoid degradation into an undesired μ
2-oxo-species (Figures 4 and 5). Degradation from exposure to moisture is accelerated for
N-HVIs possessing more sterically hindered or electron-deficient heterocyclic ligands. This degradation is pictured below for the
2-OMe-Py-HVI, in which a small sample was placed on a watch glass and degradation was monitored both qualitatively, by color change (Figure 4), and by
1H-NMR spectroscopy (Figure 5) at 5-min intervals. Partial degradation was observed after just 5 min, and complete disappearance of desired product was observed within 10 min of exposure to air inside a fume hood.
Figure 4. Degradation of 2-OMe-Py-HVI when exposed to air on a dry watch glass at various time points (photos provided by submitter)
Figure 5. Degradation of 2-MeO-Py-HVI monitored by 1HNMR at 5 min intervals
(Figure provided by submitters)
15. The submitters used the following procedure in an air- and moisture-free glovebox for the isolation of the product. The flask containing the precipitated product is evacuated and purged with argon 5 times, and the rubber septum is securely fixed to the neck with electrical tape. The flask and its contents are then transferred to a glovebox (
Note 18) for inert filtration under a
nitrogen atmosphere (Figure 6a). Contents of the flask are poured onto a 4-5 µm glass fritted filter (Figure 6b) (
Note 19), which is placed on a 250 mL filter flask, at which time suction is applied via vacuum (Figure 6c) (
Note 20). The submitters used an air- and moisture-free glovebox (<0.5 ppm each) for the isolation of the product. The flask is evacuated and purged with argon 5 times, and the rubber septum is securely fixed to the neck with electrical tape. The flask and its contents are then transferred to a glovebox (
Note 18) for inert filtration under a
nitrogen atmosphere (Figure 4a). Contents of the flask are poured onto a 4-5 µm glass fritted filter (Figure 6b) (
Note 19), which is placed on a 250 mL filter flask, at which time suction is applied via vacuum (Figure 6c) (
Note 20). The reaction flask is rinsed with diethyl ether (25 mL) (
Note 6) to remove any residual solids, and these solids are collected on the filter. The combined solids are rinsed with additional
diethyl ether (35 mL). Following filtration, the damp product is transferred via spatula to a pre-weighed, single-necked (24/40) 100 mL round-bottomed flask for drying (Figure 6d). The flask is evacuated and left under constant vacuum for 1 h to reveal the product as a free-flowing, off-white powder (4.40 g, 89%) (Notes
14 and
15). The product can then be transferred to a flame-dried product vial (20 mL) for long term storage in moisture-free conditions such as a glovebox or desiccator (
Note 14).
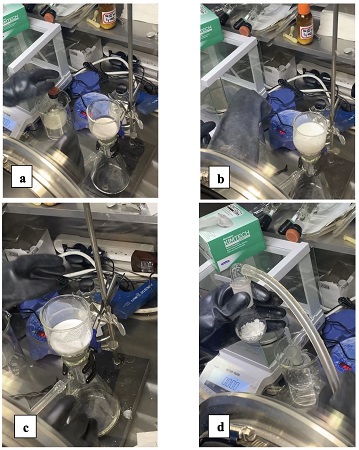
Figure 6. N-HVI transferred into glovebox in 250 mL round-bottomed flask prior to filtration; (b) solution of N-HVI suspended in solvent poured onto filter prior to application of suction; (c) N-HVI after removal of solvent via vacuum filtration and (d) N-HVI product transferred to 100 mL round-bottomed flask to further dry under vacuum
(photos provided by submitters)
16. Characterization data for
4-CF3-Py-HVI (
3):
1H NMR
pdf (500 MHz, CD
3OD) δ: 7.75 (t, 2H,
J = 7.8 Hz), 7.88 (t, 1H,
J = 7.5 Hz), 8.14 (d, 4H,
J = 6.3 Hz), 8.41 (d, 2H,
J = 7.4 Hz), 9.01 (d, 4H,
J = 6.3 Hz);
13C NMR
pdf (125 MHz, CD
3OD) δ: 121.3 (q,
J = 316.2 Hz), 121.7, 123.0 (q,
J = 271.0 Hz), 123.4 (q, J = 3.8 Hz), 132.9, 135.1, 137.1, 143.8 (q,
J = 35.0 Hz), 147.3;
19F NMR
pdf (470 MHz, CD
3OD) δ: -66.82, -80.09; IR (ATR): 3100, 3060, 3032, 1433, 1320, 1250, 1145, 1024, 840, 632. Elemental Analysis: calc'd; C: 30.17, H: 1.65, N: 3.52. Found; C: 29.65, H: 1.66, N: 3.21 (submitter's data) Due to instability and rapid decomposition, the checkers were unable to obtain purity data. Due to inherent instability, melting point was not determined.
17. A second reaction performed the same scale provided 4.43 g (90%) of the same product.
18. The glovebox used is a Vacuum Atmospheres NexGen system. A glovebox is required to obtain high yields of chemically pure
N-HVIs containing more sterically hindered or electron deficient heterocyclic ligands. The presence of trace water is known to cause rapid degradation, and therefore any exposure to atmospheric moisture during filtration or transfer for storage leads to depreciation of yield and reagent quality.
19. Due to the fine nature of the powder product, a fritted filter (150 or 250 mL) with small pore size (4-5 µm) is required to capture all solids. Use of a 60 µm pore size led to loss of a significant amount of material through the filter.
20. The vacuum used for glovebox filtration is a Welch Systems belt drive vacuum pump (8-11 mmHg).
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
Discussion
Hypervalent iodine compounds have emerged as a versatile, non-toxic, environmentally benign class of reagents class that can often serve as alternatives to transition metal-mediated transformations.
2 In particular, I(III) species serve as mild oxidants, group transfer agents, and electrophilic activators that enable a myriad of synthetic transformations. These reagents feature a central aryl iodine that shares a hypervalent 3c-4e
- bond with two X-ligands, which are most commonly either carboxylates (i.e.
PhI(OAc)2, PhI(OTFA)
2) or halogens (i.e. PhICl
2).
Over the past several years, our laboratory has been exploring a class of (bis)cationic
nitrogen-ligated I(III) reagents possessing two datively bound aromatic
nitrogen heterocycles as X-ligands, which we have termed
N-HVIs. The first examples of these reagents were reported in 1994 by Weiss,
3 but the subsequent 20 years saw almost no explorations into their synthetic utility.
4,5,6,7,8 N-HVI's undergo facile ligand exchange with nucleophiles, show enhanced oxidation potentials, and the
nitrogen heterocycles provide excellent handles for tuning reactivity and selectivity. Several groups have reported on their utility in a variety of oxidative transformations, pi-bond activation, or in accessing high-valent metal complexes, representative examples of which are shown in Figure 7. Efforts in our laboratory have found that
N-HVIs can activate alcohols for oxidative ring expansions to access medium-ring ethers,
9,10 or enable mild, equatorial-selective oxidation to the corresponding carbonyls,
11 transformations not accessible with other I(III) species. More recent studies by our group have unlocked their potential as "heterocyclic group transfer" reagents for the synthesis of diverse (heteroaryl)onium salts.
12
In light of ever-expanding suite of transformations enabled by N-HVIs, the disclosure of a detailed protocol for their synthesis and isolation that includes several useful updates to the original procedure seemed timely. The procedure above highlights the preparation of two members of this class of compounds. Procedure A is generally applied to the synthesis of N-HVI reagents with heterocyclic ligands that are (1) non-sterically hindered, (2) electron neutral, or (3) electron-rich. Procedure B is generally applied to the synthesis of N-HVI reagents with heterocyclic ligands that are (1) sterically hindered and (2) electron deficient.
Figure 7. Representative examples of transformations enabled by N-HVIs both from our lab (left hand side) and others
Following Weiss' original report,
N-HVIs can be readily accessed from commercial
PhI(OAc)2 via activation with a silyl triflate followed by addition of the heterocycle of choice.
3 This leads to precipitation of the
N-HVI as the bistriflate salt which can then be collected via filtration. A key feature in working with
N-HVIs is their moisture sensitivity, which varies depending on the steric and electronic nature of the heterocyclic ligand. There are conflicting reports regarding the stability of
N-HVIs, however we have found that, with proper technique, a wide variety of
N-HVIs can be reliably synthesized and handled either on the benchtop or using inert atmosphere conditions. In this report, we detail two strategies for the synthesis and isolation of
N-HVIs with varying levels of bench stability including both a benchtop and a glovebox isolation. Furthermore, it was found that substitution of
diethyl ether for
dichloromethane improves the isolation and subsequent stability of the
N-HVIs shown in this report. It is our aim that with this
Organic Syntheses protocol we can demystify the synthesis and handling of
N-HVIs, opening the door to more development and application of this valuable reagent class in organic synthesis.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Diacetoxyiodobenzene: Iodine, bis(acetato-κO)phenyl-; (3240-34-4)
Trimethylsilyl trifluoromethanesulfonate: Methanesulfonic acid, 1,1,1-trifluoro-, trimethylsilyl ester; (27607-77-8)
Pyridine: pyridine; (110-86-1)
2-Methoxypyridine: Pyridine, 2-methoxy-; (1628-89-3)
4-(Dimethylamino)pyridine: 4-Pyridinamine, N,N-dimethyl-; (1122-58-3)
4-(Trifluoromethyl)pyridine: Pyridine, 4-(trifluoromethyl)-; (3796-24-5)
Py-HVI, [(pyridine)2IPh](OTf)2: Iodine(2+), phenylbis(pyridine)-, 1,1,1-trifluoromethanesulfonate (1:2);(2) (156002-39-0)
4-CF3-Py-HVI, [(4-(trifluoromethyl)pyridine)2IPh](OTf)2: (3) (No CAS Registry number found)

|
Bilal Hoblos obtained his BS in chemistry from The Pennsylvania State University, Behrend Campus in 2016. He then moved to Temple University in Philadelphia, PA to pursue a PhD under the guidance of Dr. Sarah Wengryniuk. His doctoral work focuses on developing divergent total syntheses of the heliannuol family of natural products utilizing via hypervalent iodine mediated oxidative rearrangements developed in Dr. Wengryniuk's laboratory. |

|
Dr. Sarah Wengryniuk obtained her B.S. in chemistry and biology from Winthrop University in Rock Hill, SC in 2007. She received her Ph.D. in 2012 under the guidance of Prof. Don Coltart at Duke University where she was supported as an NSF Graduate Fellow. After completing postdoctoral training in the laboratory of Prof. Phil S. Baran at The Scripps Research Institute as an NIH Ruth L. Kirchstein fellow, she began her independent career at Temple University in 2015 where she is currently an assistant professor. Her laboratory works on the development of novel reverse-polarity transformations enabled by hypervalent iodine reagents. |

|
Jeff Kuethe studied chemistry at Middle Tennessee State University where he received a Bachelor of Science in 1993. He then joined the group of Professor Albert Padwa at Emory University in Atlanta, Georgia where he received a Ph.D. in 1998. He continued as a postdoctoral fellow in the group of Professor Daniel Comins at North Carolina State University before joining the Department of Process Research at Merck & Co., Inc., Rahway, New Jersey in 2000. His research interests include Process research, synthetic methodology, heterocyclic chemistry, alkaloid and natural product synthesis, and tandem transformations. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved