Org. Synth. 2022, 99, 1-14
DOI: 10.15227/orgsyn.099.0001
Synthesis of [(R)-DTBM-SEGPHOS]NiCl2 for the Enantioselective Acetal Formation from N-Propanoyl-1,3-Thiazinane-2-thione and Trimethyl Orthoformate
Submitted by Stuart C. D. Kennington, Pedro Romea,* and Fèlix Urpí*
Checked by Zhaobin Han and Kuiling Ding
1. Procedure (Note 1)
A. [(R)-DTBM-SEGPHOS]NiCl2 (1). An oven--dried single-necked 25 mL round-bottomed flask (14/23 joint), equipped with a 1.5-cm Teflon-coated magnetic stir bar, is charged with (R)-DTBM-SEGPHOS (1.00 g, 0.85 mmol, 1 equiv) (Note 2) and NiCl2 (110 mg, 0.85 mmol, 1 equiv) (Note 3), and acetonitrile (15 mL) (Note 4). A reflux condenser (14/23 joint) with a rubber septum is attached to the round-bottomed flask, and the system is purged for 5 min with N2, after which a N2-filled balloon is used to seal the system. The mixture is then heated at reflux in an oil bath for 16 h.
A Number 3 Glass filter funnel connected with a vacuum adaptor to a single-necked 500 mL round-bottomed flask (29/32 joint) is charged with Celite® (25 g) (Note 5). The Celite® is wetted with acetonitrile (70 mL) (Note 4) and allowed to settle. Whilst the reaction mixture is still warm, the contents are poured over the Celite®. Once absorbed, the Celite® is carefully washed (Note 6) with acetonitrile (300 mL) (Note 4) until the color completely passes to the round-bottomed flask. The filtrate is concentrated on a rotary evaporator (30 °C, 12 mmHg). The resulting solid is dissolved in dichloromethane (20 mL) (Note 7) and transferred to a vial (20 mL). The solution is concentrated on a rotary evaporator (30 °C, 12 mmHg) and the resulting solid is broken up with a spatula and dried on a high vacuum line (0.1 mmHg) for 4 h giving the title compound 1 (1.10 g, 0.84 mmol, 99%) (Note 8) as a fine dark green-black powder.
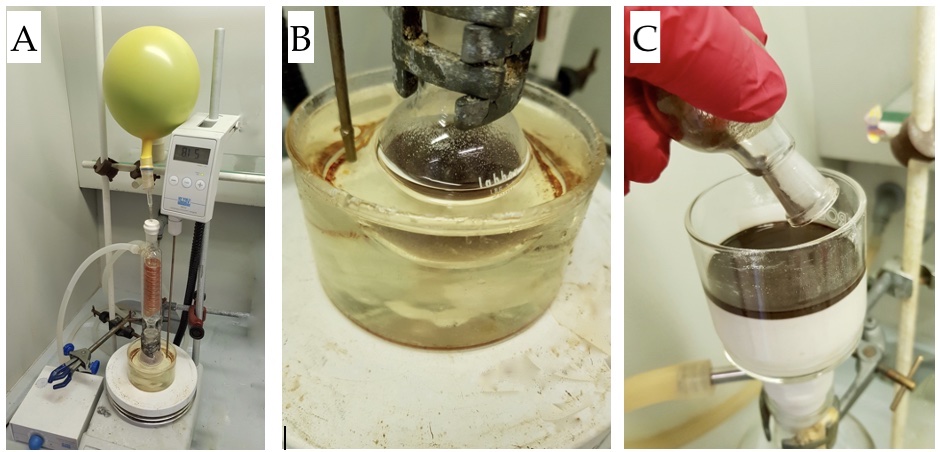
Figure 1. A) Reaction set-up, B) final color, and C) Celite® filtration (photos provided by submitters)
B. N-[(S)-(3,3-Dimethoxy-2-methylpropanoyl)]-1,3-thiazinane-2-thione (2). An oven dried single-necked 50 mL round-bottomed flask (14/23 joint), equipped with a 2-cm Teflon-coated magnetic stir bar, is charged with N-propanoyl-1,3-thiazinane-2-thione (1.89 g, 10 mmol, 1 equiv) (Note 9) and 1 (196 mg, 0.15 mmol, 1.5 mol%). The round-bottomed flask is sealed with a rubber septum and purged with N2 for 5 min, after which a N2-filled balloon is attached to the system. Freshly distilled dichloromethane (20 mL) (Note 7) is added to the mixture, followed by trimethyl orthoformate (1.3 mL, 12 mmol, 1.2 equiv) (Note 10). The resultant mixture is stirred and cooled to -40 °C with an acetonitrile/liquid N2 bath. Then, triethylsilyl triflate (3.2 mL, 14 mmol, 1.4 equiv) (Note 11) is added and the mixture is stirred for 5 min. Finally, freshly distilled 2,6-lutidine (1.75 mL, 15 mmol, 1.5 equiv) (Note 12) is added and the resultant mixture is stirred for 2 h at -40 °C.
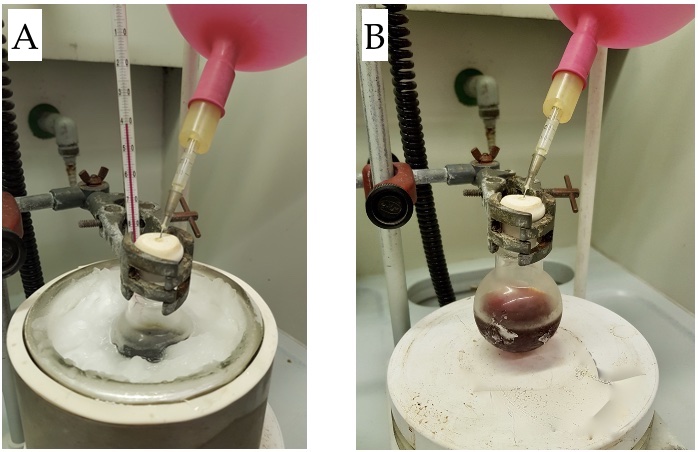
Figure 2. A) Reaction set-up and B) final appearance (photos provided by submitters)
The reaction mixture is quenched with saturated NH4Cl aqueous solution (10 mL), and the mixture is transferred to a 250 mL separating funnel. The round-bottomed flask is washed with dichloromethane (2 × 25 mL) (Note 13), which is then transferred to the separating funnel. Deionized water (2 × 25 mL) is used to wash the round-bottomed flask and added to the separating funnel. After shaking vigorously, the lower organic layer is collected, and the remaining aqueous layer is further extracted with dichloromethane (50 mL) (Note 13). The combined organic extracts are dried over MgSO4 (30 g) (Note 14) and filtered into a 250 mL round-bottomed flask (29/32 joint); the remaining MgSO4 (Note 14) is washed with dichloromethane (50 mL) (Note 13) and filtered. The combined filtrates are concentrated on a rotary evaporator (25 °C, 12 mmHg) to produce a yellow-brown oil.
The residue is then submitted to flash column chromatography using a 4.5 cm diameter column with a length of 25 cm of silica gel (60 Å) (Note 15). This is first compacted with 95:5 petroleum ether/ethyl acetate (500 mL) (Note 16), and the surface levelled. The residue is dissolved in dichloromethane (5 mL) (Note 13) and transferred onto the compacted column with a pipette. The round-bottomed flask is washed with additional dichloromethane (2 × 2 mL) (Note 13) and the washings added to the column. Once adsorbed, thick sand is added to protect the silica. The column is eluted with a 10/1 mixture of petroleum ether and ethyl acetate (ca 2000 mL) (Note 16) until all of the yellow color has left the column and the eluent runs clear. All pure tubes are collected in a 500 mL round-bottomed flask and concentrated on a rotary evaporator (35 °C, 12 mmHg). Neat dichloromethane (2 ´ 25 mL) (Note 13) is added and the resultant solution transferred to a vial (20 mL) where it is concentrated on a rotary evaporator (35 °C, 12 mmHg). The resultant solid is kept under high vacuum (0.1 mmHg) at room temperature for 4 h to afford pure product 2 (2.38 g, 90% yield) as a yellow solid (Note 17).
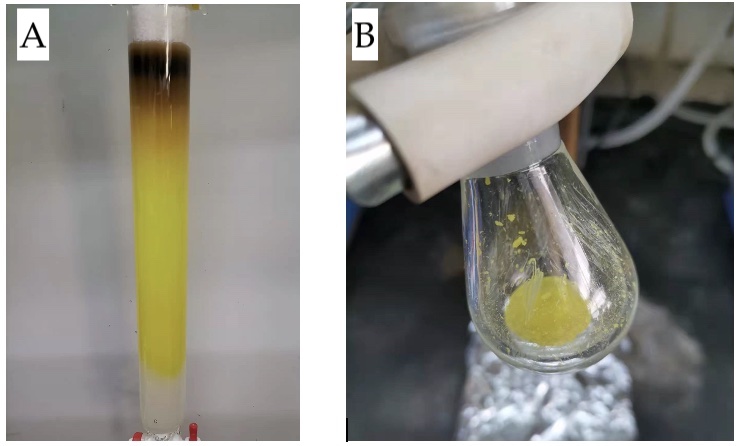
Figure 3. A) Chromatographic column (photo provided by submitters), and B) final product (photo provided by checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
(R)-DTBM-SEGPHOS,
NiCl2,
acetonitrile,
Celite®,
dichloromethane,
trimethyl orthoformate,
triethylsilyl triflate,
2,6-lutidine,
NH4Cl, anhydrous
MgSO4, petroleum ether,
ethyl acetate,
1,3,5-trimethoxybenzene, and
CDCl3.
2.
(R)-DTBM-SEGPHOS (> 99%) was purchased from TCI Europe and used as received. The checkers purchased
(R)-DTBM-SEGPHOS (>99.0%) from Tokyo Chemical Industry Co., Ltd. and the material was used as received.
3.
NiCl2 (99%) was purchased from Sigma-Aldrich and used as received. The checkers purchased nickel chloride anhydrous (>98.0%) from Tokyo Chemical Industry Co., Ltd. and the material was used as received
4.
Acetonitrile (99%) was purchased from VWR International and used as received. The checkers purchased
acetonitrile (99%) from Sinopharm Chemical Reagent Co., Ltd. and the solvent was used as received.
5.
Celite@ was purchased from Fluorochem and used as received.
6. It is important to wash the
Celite® surface carefully so that only minimal stirring of the solid occurs.
7.
Dichloromethane (99%) was purchased from Acros Organics and was freshly distilled over CaH
2. The checkers purchased
dichloromethane (99%) from Sinopharm Chemical Reagent Co., Ltd. and it was freshly distilled over CaH
2.
8. A second reaction on identical scale provided 1.09 g (98%) of the product
1.
[(R)-DTBM-SEGPHOS]NiCl2 (
1) has the following physical and spectroscopic properties: mp 249-251 °C; IR (film): 2956, 1438, 1410, 1393, 1227, 1178, 1138, 1116, 1052, 1006, 807, 639 cm
-1; HRMS (+ESI)
m/z calcd for C
74H
100ClNiO
8P
2 [M-Cl]
+ 1271.5930, found 1271.5938; Calcd for C
74H
100Cl
2NiO
8P
2: C, 67.89; H, 7.70. Found: C, 68.44; H, 7.93.
9. Preparation of
N-propanoyl-1,3-thiazinane-2-thione has been reported in
Org. Synth.
2021,
98, 374-390.
10.
Trimethyl orthoformate (99%) was purchased from Sigma-Aldrich and used as received. The checkers purchased
trimethyl orthoformate (>98.0%) from Tokyo Chemical Industry Co., Ltd. and used it as received.
11.
Triethylsilyl triflate (> 98%) was purchased from Fluorochem and used as received. The checkers purchased
triethylsilyl triflate (>98.0%) from Tokyo Chemical Industry Co., Ltd. and used it as received. Importantly,
triethylsilyl triflate must be new and fresh. It is imperative that it appear as a clear liquid.
12.
2,6-Lutidine (98%) was purchased from Sigma-Aldrich and freshly distilled over CaH
2. The checkers purchased
2,6-lutidine (98%) from Aladdin and freshly distilled the material over CaH
2.
13.
Dichloromethane (99%) was purchased from Acros Organics and used as received. The checkers purchased
dichloromethane (99%) from Sinopharm Chemical Reagent Co., Ltd. and used it as received.
14. Anhydrous
MgSO4 was purchased from Panreac and used as received. The checkers purchased anhydrous
MgSO4 (99%) from Sinopharm Chemical Reagent Co., Ltd. and used it as received.
15. Silica gel was purchased from Sigma-Aldrich and used as received. The checkers purchased silica gel (SiliaFlash P60, particle size: 40-63 µm, pore size 60 Å ) from SILICYCLE and it was used as received.
16. The checkers purchased petroleum ether (bp 60-90 °C, 99%) and
ethyl acetate (99%) from Tansoole and used it as received.
17. A second reaction on identical scale provided 2.36 g (90%) of the product
2.
N-[(S)-(3,3-Dimethoxy-2-methylpropanoyl)]-1,3-thiazinane-2-thione (
2) has the following physical and spectroscopic properties: R
f 0.55 (Petroleum ether/
ethyl acetate = 2/1); chiral HPLC (Chiralcel AD-3, 3%
i-PrOH in hexane, flow 1 mL min
-1, 254 nm] R
t 13.0 min [R
t minor 13.7 min], 99%
ee; mp 57-58 °C; [α]
D23 +438 (
c 1.00, CHCl
3); IR (film): 2938, 2831, 1710, 1463, 1431, 1375, 1345, 1264, 1175, 1139, 1099, 1051, 993, 709 cm
-1;
1H NMR
pdf (400 MHz,
CDCl3) δ: 1.30 (d,
J = 6.4 Hz, 3H), 2.12-2.36 (m, 2H), 2.95-3.15 (m, 2H), 3.30 (s, 3H), 3.33 (s, 3H), 3.48 (ddd,
J = 13.2, 9.6, 4.0 Hz, 1H), 3.87-4.01 (m, 1H), 4.13 (dt,
J = 13.2, 4.8 Hz, 1H), 4.41 (d,
J = 8.0 Hz, 1H);
13C NMR
pdf (100.6 MHz,
CDCl3) δ: 13.9 (CH
3), 22.6 (CH
2), 31.9 (CH
2), 45.9 (CH), 47.8 (CH
2), 52.1 (CH
3), 55.3 (CH
3), 107.4 (CH), 181.6 (C), 201.0 (C); HRMS (+ESI)
m/z calcd for C
10H
17NO
3NaS
2 [M +Na]
+ 286.05421, found 286.05340. The purity of product
2 was determined using
1H QNMR
pdf analysis.
1H QNMR
pdf was performed using a mixture of
2 (22.1 mg) and
1,3,5-trimethoxybenzene (12.3 mg) (Alfa Aesar, 99% purity, white solid, as an internal standard) in
CDCl3. The purity was calculated according to standard method as 99.2 wt%.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The enantioselective and catalytic construction of carbon-carbon bonds from enolates has attracted much attention in recent years.
2 Indeed, Evans,
3 Shibasaki,
4 and Trost
5 have described insightful approaches taking advantage of privileged scaffolds and chiral nickel(II), copper(II), or zinc complexes. Regardless of the outcome of such transformations, availability and easily handled catalysts are important issues to make them useful to be applied to the asymmetric synthesis of natural products.
In this context, we have recently reported the reaction of
N-acyl-1,3-thiazinane-2-thione with a range of electrophiles in the presence of 1-10 mol% of
[(R)-DTBM-SEGPHOS]NiCl2.
6,7 Evidence suggests that such a complex is activated
in situ with TESOTf to produce the true catalytic species, [(
R)-DTBM-SEGPHOS]Ni(OTf)
2, which coordinates with the
N-acyl thiazinanethione to make possible the Cα deprotonation with
2,6-lutidine (Scheme 1). In turn, TESOTf is also necessary to generate
in situ the electrophilic reacting species from methyl orthoformate, acetals, ketals, or benzhydryl methyl ethers. Then, the resultant chiral nickel(II) enolate approaches the electrophilic intermediate through an open transition state to produce the corresponding adduct containing a new carbon-carbon bond and up to two new stereogenic centers (Scheme 1).
Scheme 1. Enantioselective carbon-carbon bond forming reactions from N-acyl-1,3-thiazinane-2-thiones and [(R)-DTBM-SEGPHOS]NiCl2
Such a process is successful and provides alkylated and aldol-like products in high yields and excellent stereocontrol. Indeed, aside from the 3,3-dimethoxy derivative described above, it is possible to obtain the adducts represented in Scheme 2 as a single enantiomer (ee ≥ 96%) in good to high yields.
Scheme 2. Enantioselective carbon-carbon bond forming reactions from N-propanoyl-1,3-thiazinane-2-thione catalyzed by [(R)-DTBM-SEGPHOS]NiCl2
Finally, the thiazinanethione scaffold can be easily removed under mild experimental conditions to afford enantiomerically pure alcohols, esters, or amides as shown in Part A of Scheme 3. Interestingly, the thiazinanethione may also act as a coupling reagent and permits the synthesis of a diastereomerically pure
N-acyl amino acid by simple addition of methyl (
S)-leucinate to the corresponding adduct in 89%yield (Part B Scheme 3).
8Scheme 3. Removal of the thiazinanethione scaffold
Appendix
Chemical Abstracts Nomenclature (Registry Number)
[(R)-DTBM-SEGPHOS]: Phosphine, 1,1'-(4R)-[4,4'-bi-1,3-benzodioxole]-5,5'-diylbis[1,1-bis[3,5-bis(1,1-dimethylethyl)-4-methoxyphenyl]-; (566940-03-2)
N-Propanoyl-1,3-thiazinane-2-thione: 1-Propanone, 1-(dihydro-2-thioxo-2H-1,3-thiazin-3(4H)-yl)-; (2138126-72-2)
Trimethyl orthoformate: Methane, trimethoxy-; (149-73-5)
Triethylsilyl triflate: Methanesulfonic acid, 1,1,1-trifluoro-, triethylsilyl ester; (79271-56-0)
2,6-Lutidine: Pyridine, 2,6-dimethyl-; (108-48-5)
N-[(S)-3,3-Dimethoxy-2-methylpropanoyl]-1,3-thiazinane-2-thione: 1-Propanone, 1-(dihydro-2-thioxo-2H-1,3-thiazin-3(4H)-yl)-3,3-dimethoxy-2-methyl-, (2S)-; (2) (2270858-10-9)

|
Stuart C. D. Kennington, born in 1992 in Cambridgeshire, England, received his MChem degree from the University of Warwick in 2015. He completed his Ph.D. thesis under the supervision of Prof. Fèlix Urpí and Pedro Romea in 2020 at the University of Barcelona with a FI scholarship from the Generalitat de Catalunya. His research focuses on new catalyzed asymmetric synthesis methodologies and their application to the total synthesis of natural products. |

|
Pedro Romea completed his B.Sc. in Chemistry at the University of Barcelona and followed Ph.D. studies from 1987 to 1991 under the supervision of Professor Jaume Vilarrasa at the same University of Barcelona. Then, he joined the group of Professor Ian Paterson at the University of Cambridge (UK), where he participated in the total synthesis of oleandolide. Back to the University of Barcelona, he became Associate Professor in 1993. His research interests have focused on the development of new synthetic methodologies and their application to the stereoselective synthesis of naturally occurring molecular structures. |

|
Fèlix Urpí received his B.Sc. in Chemistry at the University of Barcelona and completed Ph.D. studies under the guidance of Professor Jaume Vilarrasa at the University of Barcelona in 1988. He then worked as a NATO postdoctoral research associate in titanium enolate chemistry with Professor David A. Evans, at Harvard University in Cambridge, MA. He moved back to the University of Barcelona, and he became Associate Professor in 1991, where he holds a chair of Full Professor in Organic Chemistry since 2017. His research interests have focused on the development of new synthetic methodologies and their application to the stereoselective synthesis of naturally occurring molecular structures. |

|
Dr. Zhaobin Han received his B.S. degree in chemistry from Nanjing University in 2003. He received his Ph.D. degree from Shanghai Institute of Organic Chemistry under the supervision of Prof. Kuiling Ding and Prof. Xumu Zhang in 2009, working on development of novel chiral ligands for asymmetric catalysis. Now he is an associate professor in the same institute and his current research interests focus on the development of efficient catalytic methods based on homogeneous catalysis. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved