Org. Synth. 2022, 99, 29-37
DOI: 10.15227/orgsyn.099.0029
Preparation of N-Formylamides by Oxidative Cleavage of N-Acylaminoacids
Submitted by Nick Kilenyi
1*Checked by Miran Lemmerer, Manuel Schupp, and Nuno Maulide
1. Procedure (Note 1)
A. N-Formylbenzamide (1). A 2 L three-necked flask equipped with a 4 cm oval magnetic stirrer bar, a stoppered pressure-equalizing addition funnel, an internal thermometer, and a reflux condenser (Figure 1) is charged with hippuric acid (71.6 g, 0.40 mol, 1.00 equiv), dichloromethane (400 mL), water (250 mL), copper(II)sulfate pentahydrate (4.99 g, 20.0 mmol, 5 mol%), and silver(I)nitrate (3.40 g, 20.0 mmol, 5 mol%) (Note 2).
The mixture is left open to the atmosphere and is heated to 40 °C (internal) with an external silicone oil bath, after which a solution of ammonium persulfate (192 g, 0.840 mol, 2.10 equiv) in water (500 mL) is added dropwise (2 drops/sec) via the addition funnel with stirring at approximately 800 rpm. Within 1-2 min the reaction begins to effervesce and undergoes a mild exotherm (Note 3) (Figure 2), at which point the heating bath is removed. The addition, which requires about 1.5 h, is adjusted to maintain a gentle reflux without external heating (around 3 drops/second) (Note 4). Stirring is continued for 2 h after the addition is complete. Towards the end of the addition time, a small amount of an insoluble brown resin forms on the walls of the flask (Figure 3).
Figure 1. Apparatus set-up (photo provided by submitter)
Figure 2. Reaction at beginning of addition (photo provided by checker)
Figure 3. Reaction at completion of addition (photo A provided by submitter and photo B provided by checker)
The reaction mixture is allowed to cool to room temperature (21 °C) and filtered from the resin on a 13 cm Por. 3 sinter. The flask and retained solids are then rinsed with additional dichloromethane (2 x 50 mL). The combined reaction mixture and washes are transferred to a 2 L separating funnel and the lower, organic phase is decanted. The aqueous phase is extracted with dichloromethane (4 x 50 mL), which is added to the original organic phase. The combined organic phases are dried over sodium sulfate (20 g) and filtered through a fluted filter paper. The drying agent is rinsed with dichloromethane (2 x 50 mL), the combined filtrates are united in a 1 L flask and evaporated to dryness (40 °C at 500 mmHg to 75 mmHg) (Note 5) (Figure 4).
The crystalline yellow solid is slurried in ethyl acetate (100 mL) in a 250 mL beaker with a 3 cm cylindrical stirrer bar. The suspension is heated to boiling (77 °C for ethyl acetate) for 1 min with an external silicone oil bath and is then allowed to cool to room temperature (21 °C) while stirring gently (140 rpm). Finally, the beaker is immersed in an ice-water bath for 10 min to complete the crystallization. The solids are collected by filtration on a 6 cm, Por. 2 sinter, dried extensively using a water aspirator, and washed with cold (0 °C) ethyl acetate (4 x 10 mL). After air-drying the material to constant weight, pure N-Formylbenzamide (1) (28.6 g, 48%) is obtained (Note 6). The filtrate is evaporated to dryness and the solid residue recrystallized from ethyl acetate to provide an additional 2.3 g of (1) with equal purity, bring the combined yield to 30.9 g (52%) (Notes 7 and 8) (Figure 5).
Figure 4. Crude product before recrystallization (photo provided by submitter)
Figure 5. Recrystallized product (photo provided by checker)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
hippuric acid,
dichloromethane,
water,
copper(II)sulfate pentahydrate,
silver(I)nitrate,
ammonium persulfate,
ethyl acetate,
sodium sulfate, and
CDCl3.
2. All solvents and reagents were obtained commercially from Fischer Scientific (
DCM, analytical grade), Fluorochem (
AgNO3,
hippuric acid,
CuSO4∙5H2O) and Alfa Aesar (
(NH4)2S2O8), and they were used as received. TLC was performed on aluminum-backed silica 60A plates (Merck).
3. No increase in temperature is observed, but the solvent continues to boil without heating.
4. The amount of suspended starting material steadily diminishes as the reaction proceeds, and the lower phase of reaction mixture develops a yellow-to-pale beige color. The upper aqueous layer remains pale blue due to the copper(II) ion.
5. Crystallization is frequently observed during evaporation.
6. Product (
1) possesses the following properties: Colorless needles, mp: 111 °C (lit
2,3 mp: 112 °C). TLC (Figure 6): Single spot at R
f 0.66 in 1:1 heptane/
ethyl acetate. Purple fluorescence when viewed under 254 nm UV light.
1H NMR
pdf (400 MHz,
CDCl3) δ: 7.55 (t,
J = 7.5 Hz, 2H), 7.66 (t,
J = 7.4 Hz, 1H), 7.91 (d,
J = 7.8 Hz, 2H), 9.12 (br, 1H), 9.38 (d,
J = 9.7 Hz, 1H).
13C NMR
pdf (100 MHz, DEPT,
CDCl3) δ: 128.0, 129.3, 131.3 (C
q), 134.1, 163.5, 166.4 (C
q). IR (thin film): 570, 673, 698, 750, 803, 884, 999, 1026, 1059, 1077, 1101, 1156, 1185, 1206, 1234, 1250, 1323, 1363, 1439, 1457, 1501, 1579, 1598, 1647, 1669, 1682, 1724, 3269 cm
-1. HRMS (ESI
+): exact mass calculated for (C
8H
7NO
2Na) required
m/z 172.0374, found
m/z 172.0369. Purity was assessed as 96.8% by qNMR
pdf using 107.2 mg of the product and
1,3,5-trimethoxybenzene (149.15 mg) as an internal standard. The submitter established purity by CHN analysis: Calc. for C
8H
7NO
2: C 64.4% H 4.7% N 9.4%; Found C 64.21% H 4.74% N 9.38%.
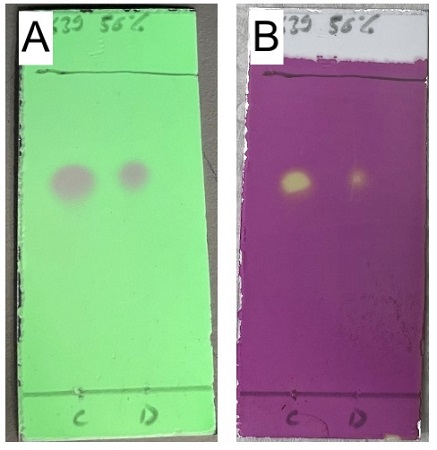
Figure 6. TLC analysis of product with visualization A) by UV, and B) staining performed with KMnO4 solution followed by heating (photos provided by checker)
7. The checkers performed a second reaction on half scale, yielding 15.7 g (53%) of the product.
8. The submitter reports that a third crop of crystals can be obtained from the evaporated filtrate by recrystallization with smaller amounts of
ethyl acetate, however this was not attempted by the checkers.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
N-Formylamides are versatile intermediates for the preparation of pharmacologically-preferred 1,2,4-triazole ring system by the Einhorn-Brunner reaction.
3,4 They are also important as starting materials for the rather inaccessible enamide and dienamide functional groups, which are found in several highly biologically-active natural products such as the antibiotics crocacin A
5 and CJ-15,801,
6 and the lituarines.
7 Until recently, these compounds could only be obtained by inconvenient and often low-yielding routes from amides by conversion of the amide to the
N-hydroxymethyl derivative with formaldehyde, followed by oxidation,
8 by
N-formylation with unstable and commercially-unavailable
N,
N-diformylacetamide,
9 or by reaction of an amide anion with
N-formylbenzotriazole.
10 The procedure described above exemplifies a shorter and much more convenient route reported by Huang and colleagues,
11 in which an
N-acylamino acid is oxidatively decarboxylated to the
N-formyl derivative with
ammonium persulfate in
water with catalytic silver(I) and copper(II) ions. The original procedure used three equivalents of persulfate and 20 mol% of the metal ions in
water under strictly oxygen-free conditions and was performed on only 0.4 mmol scale. We have found that the reaction is completely insensitive to oxygen, and the reaction can even be run in an open flask. Furthermore, we have found that the oxidation requires only the stoichiometric amount of persulfate (2 equiv) and lower catalytic amounts of copper(II) and silver(I) (5 mol% or less) for the reaction to run to completion. On a large scale (0.1 mol and above), it is advantageous to run the reaction in a two-phase
water-
dichloromethane or
water-
ethyl acetate mixture as this solvent mixture assists in keeping the starting material and product in solution, and controls foaming. The solvent at reflux also prevents the exotherm, when performed on a mole scale or larger, from getting out of hand. We believe this modification of the Huang procedure to be the most convenient and cost-effective route to
N-formylamides, especially when working on a large scale. However, it should be noted that the method has a significant limitation in that
N-acylaminoacids bearing a substituent on the nitrogen often give poor yields due to competing cleavage of the likely iminium ion intermediate. Thus, an attempt to make the
N-methyl analogue of (
1) by oxidative cleavage of
N-methylhippuric acid gave a 1:1 mixture of the desired product and
N-methylbenzamide.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Ammonium persulfate; (7727-54-0)
Copper(II)sulfate pentahydrate; (7758-99-8)
N-Formylbenzamide; (1) (4252-31-7)
Hippuric acid; (495-69-2)
Silver(I)nitrate; (7761-88-8)
Sodium sulfate; (7757-82-6)

|
Steven Nicholas (Nick) Kilenyi received his chemical training at Imperial College, London and the University of East Anglia. Following a NATO post-doctoral fellowship at the University of Pennsylvania he spent many years working in drug discovery at Pfizer in Sandwich and Sanofi in Brussels. More recently, he taught Chemistry at International Schools in Chengdu and Beijing before accepting his current position in 2017 on the picturesque North coast of Cornwall. |

|
Miran Lemmerer is currently conducting his Ph.D. thesis research under the supervision of Prof. Dr. Nuno Maulide, focusing on the development of novel methodology based on iminium ions. |

|
Manuel Schupp conducted his M.Sc. studies under supervision of Prof. Dr. Hans-Achim Wagenknecht (Karlsruhe Institute of Technology) and Prof. Dr. Andrew G. Myers (Harvard University) before joining the group of Prof. Dr. Nuno Maulide (University of Vienna & CeMM) as a Ph.D. student in 2019. His studies focus on the total synthesis of immunosuppressive natural products and organoselenium-catalyzed rearrangements. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved