Org. Synth. 2025, 102, 203-216
DOI: 10.15227/orgsyn.102.0203
Iodine-Catalyzed Stereospecific anti-Diamination of trans-β-Methylstyrene
Submitted by Takeshi Sugiyama, Chiyuki Naito, and Satoshi Minakata*
Checked by Shun Kawano, Juri Sakata, and Hidetoshi Tokuyama
1. Procedure (Note 1)
N,N'-((1R*,2S*)-1-Phenylpropane-1,2-diyl)bis(2-nitrobenzenesulfonamide) 1. A 500-mL three-necked round-bottomed flask equipped with a football-shaped Teflon-coated magnetic stir bar (Note 2) is charged with nosylamide (Note 3) (8.50 g, 42.0 mmol, 2.10 equiv) and iodine (Note 4) (508 mg, 2.00 mmol, 10.0 mol%), sequentially. The flask is fitted with a thermometer and drying tube (Note 5), and clamped on a digitally controlled stirrer. Acetonitrile (Note 6) (200 mL) is measured in a graduated cylinder and poured into the reaction flask via side neck, and the reaction mixture is stirred at 500 rpm for 10 min (Figure 1A). NaOCl·5H2O (Note 7) (7.00 g, 42.0 mmol, 2.10 equiv) is weighed in a beaker with a plastic spatula and added to the flask via the middle neck using the spatula, affording the yellow suspension (Figure 1B). Then, trans-β-methyl styrene (Note 8) (2.40 g, 20.0 mmol, 1.00 equiv) is added to the reaction flask via side neck using a syringe. The flask is placed into an oil bath pre-heated to 40 ℃ (Figure 2).
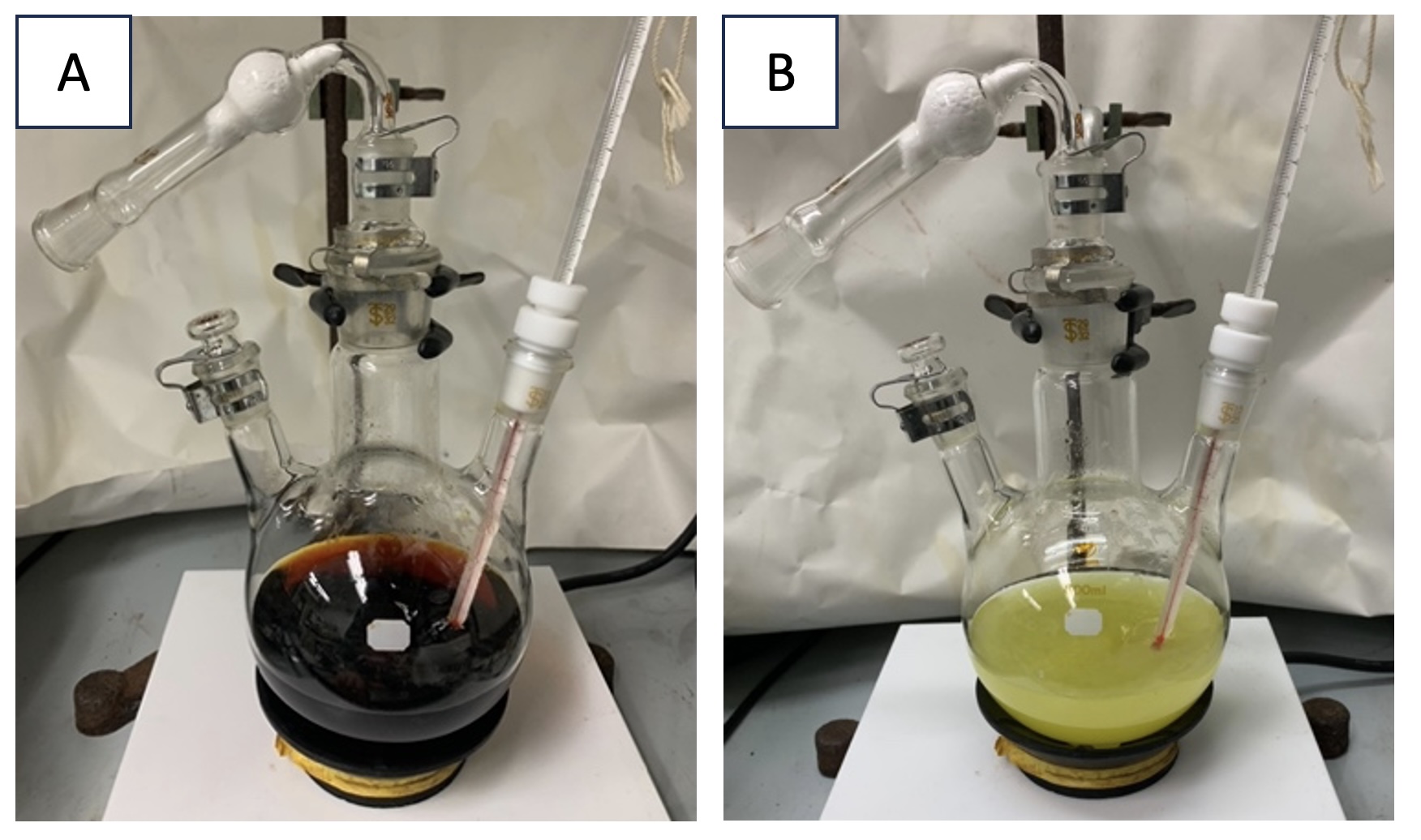
Figure 1. A. Mixture of nosylamide and iodine in MeCN; B. after adding NaOCl·5H2O (photos provided by checkers)
Figure 2. Reaction set-up for the diamination (photo provided by checkers)
The yellow solution is stirred at 500 rpm for 12 h at 40 ℃ (internal temperature), then it is warmed to 75 ℃ and stirred at 500 rpm for 6 h (Note 9 and 10). The resulting cloudy yellow solution is allowed to cool to room temperature (22 ℃) (Figure 3A). Water (100 mL) and EtOAc (Note 11) (180 mL) are added to the reaction mixture, and the resulting two-phase solution is transferred to a 1-L separatory funnel. Additional EtOAc (20 mL) is used to transfer residual oil from the flask to the separatory funnel. The two-phase solution is partitioned, and the organic phase is washed with brine (100 mL) and aqueous solution of sodium thiosulfate (100 mL) (Note 12). The organic phase is dried over anhydrous sodium sulfate (Note 13) (40 g). The sodium sulfate is removed by filtration through a plastic funnel with a cotton plug. After rinsing the residue in the funnel with EtOAc (3 x 10 mL), the filtrate and washings in 1-L flask are concentrated on a rotary evaporator under reduced pressure (40 ℃, 40 mm Hg), and the resulting residual oil is dried in vacuo for 1 h (room temperature, 22 ℃, <1 mm Hg) to afford a crude product as a yellow solid (Figure 3B, Note 14).
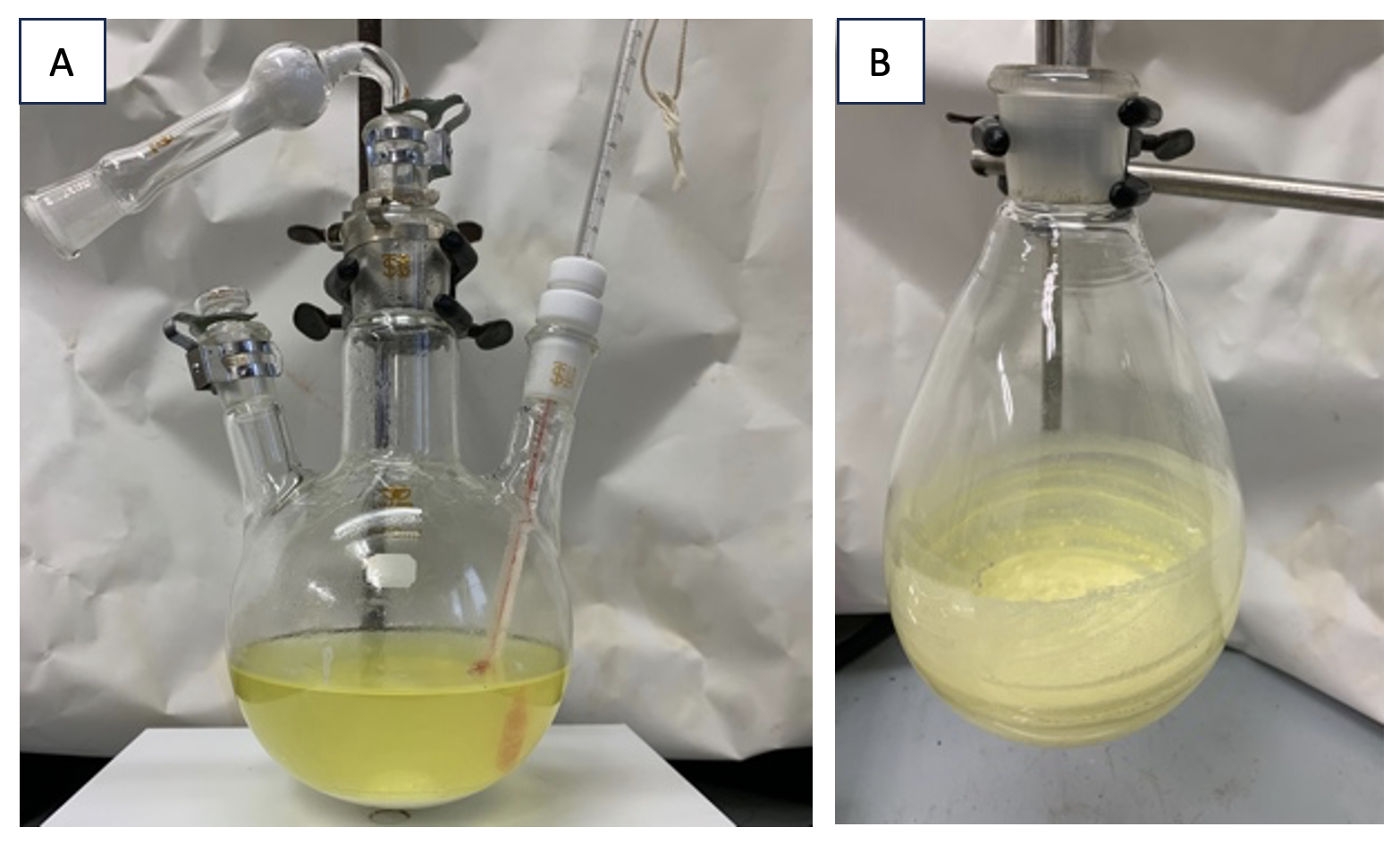
Figure 3. A. The reaction mixture after stirring for 18 h; B. the crude yellow solid. (photos provided by checkers)
Diethyl ether (Note 15) (200 mL) is poured into the crude product (Figure 4A), and the solid is ground with a spatula (Note 16) (Figure 4B). The suspension is sonicated (Note 17) for 30 min and the precipitate is collected by vacuum filtration (Note 17, Figure 5). Additional Et2O (3 x 10 mL) is used to transfer residual solid from flask to the filter funnel. The solid on the filter paper is rinsed with Et2O (100 mL), water (50 mL) and again Et2O (100 mL). The resulting solid is dried in vacuo for 10 h (40 ℃, <1 mm Hg) in a desiccator with phosphorus pentoxide (Note 18) to yield the desired product (1) as a white solid (7.20 g, 69%) (Notes 19 and 20) (Figure 6).
Figure 4. A. The addition of Et2O to the crude solid: before grinding; B. during grinding (photos provided by checkers)
Figure 5. A. The filtration set-up for collecting the diaminated product: before filtration; B. during filtration (photos provided by checkers)
Figure 6. Purified N,N'-((1R*,2S*)-1-phenylpropane-1,2-diyl)bis(2-nitrobenzenesulfonamide)(1) (photo provided by checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/about/governance/committees/chemical-safety.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
nosylamide,
iodine,
acetonitrile,
NaOCl·5H2O,
trans-β-Methyl styrene,
EtOAc,
sodium thiosulfate,
Et2O, and
phosphorus pentoxide, as well as the proper procedures for weighing and addition of
NaOCl·5H2O.
Caution! NaOCl·5H2O must be kept away from contact with metals and fabrics. Avoid using weighing paper or metal spatula, and instead, use a glass or plastic tray and spatula for weighing. Additionally, due to its hygroscopicity and low melting point, it is recommended to weigh quickly after taking it from refrigerator. The details of property and safety assessment of NaOCl·5H2O are described in the literature.22. 500-mL three-necked (2 x 15/25 and 29/32) round-bottomed flask is used. Stir-bar size is 40 mm x 20 mm x 18 mm.
3.
2-nitrobenzenesulfonamide (>98%) was purchased from Tokyo Chemical Industry Co., Ltd. and used as received.
4.
Iodine (>99.8%) was purchased from FUJIFILM Wako Pure Chemical Corporation as a granular
iodine and used after grinding in a mortar with a pestle.
5. The drying tube is 20-30 mm i.d. and 90 mm length, and filled with a calcium chloride, and the open end of drying tube is blocked with cotton and the drying tube is connected to the neck of reaction flask via the ground glass joint. The other neck of flask is capped with glass stopper.
6.
Acetonitrile (>99.5%) was purchased from FUJIFILM Wako Pure Chemical Corporation and used as received.
7.
NaOCl·5H2O (Wako 1
st Grade) was purchased from FUJIFILM Wako Pure Chemical Corporation and used as received.
8.
trans-β-Methyl styrene (>97%) was purchased from Tokyo Chemical Industry Co., Ltd. and used as received. It contains 4-
tert-butylcatechol as a stabilizer but does not affect the reaction.
9. Elevating reaction temperature to 75 ℃ is necessary to convert the
N-Ts aziridine intermediate into the desired product. As the reaction proceeds, a yellow clear solution turns cloudy to form a white solid (likely NaCl) (Figure 3 A).
10. The progress of the reaction is monitored by TLC analysis on silica gel with 70%
ethyl acetate in
hexane used as an eluent. The plate is visualized using a UV lamp (254 nm). The starting materials,
trans-β-methyl styrene and
nosylamide have R
f = 0.86 and R
f = 0.54 and the diaminated product has R
f = 0.64 (Figure 7).
Figure 7. TLC of the crude reaction mixture (photo provided by checkers)
11.
Ethyl acetate (>99%) was purchased from NACALAI TESQUE, INC. and used as received.
12. The concentration of
sodium thiosulfate aqueous solution is 0.5 M.
Sodium thiosulfate pentahydrate (>99%) was purchased from FUJIFILM Wako Pure Chemical Corporation and used as received.
13. Anhydrous
sodium sulfate (>98.5%) was purchased from NACALAI TESQUE, INC. and used as received.
14. The TLC of the mixture after work up is monitored following by the same conditions described in
Note 10 (Figure 7).
Figure 8. TLC of the reaction mixture after work up (photo provided by checkers)
15.
Diethyl ether (>99.5%) was purchased from Showa Ether, INC. and used as received.
16. The yellow crude product should be ground with a spatula as finely as possible in
Et2O. In addition to this operation, sonication is carried out to grind the solid and dissolve undesired compounds into
Et2O.
17. Sonication is conducted using a BRANSONIC
® ULTRASONIC CLEANER, Model 2510J-MTH at power of 100 W. The precipitate is collected by suction filtration through a filter paper (No. 4) using a 4.0 cm diameter Kiriyama funnel. A
CO2/
acetone trap was inserted between the Kiriyama funnel and the pump to trap
Et2O (Figure 5).
18.
Phosphorus pentoxide (>98%) was purchased from FUJIFILM Wako Pure Chemical Corporation and used as received.
19. Yields both of two runs were obtained as 69% by checkers. The purity of diaminated product
1 was determined to be 99.2% by qNMR
pdf using
1,3,5-trimethoxybenzene (Aldrich Chemical Co., Inc., >99.9%) as the internal standard.
20. Characterization of
1: mp: 198.8-200.2 ℃ (
acetone);
1H NMR
pdf (600 MHz, DMSO-
d6) δ: 8.59 (d,
J = 9.0 Hz, 1H), 7.97 (d,
J = 9.0 Hz, 1H), 7.79-7.70 (m, 3H), 7.65-7.59 (m, 3H), 7.55-7.49 (m, 2H) 7.09-7.08 (m, 2H), 6.87-6.86 (m, 3H), 4.33 (dd,
J = 9.6, 9.6 Hz, 1H), 3.73-3.72 (m, 1H), 1.11 (d,
J = 6.0 Hz, 3H).;
13C NMR
pdf (150 MHz, DMSO-
d6) δ: 146.9, 146.9, 138.4, 133.6, 133.5, 132.9, 132.6, 132.0, 129.9, 129.4, 127.4, 127.1, 124.3, 123.9, 61.9, 54.0, 19.1. (Two sp
2 carbon signals were not observed because of overlapping); IR (ATR): 3320, 1528, 1451, 1363, 1348, 1167, 1054, 909, 858, 780, 729 cm
-1.; HRMS (ESI):
m/z calc'd. for (C
21H
21N
4O
8S
2) 521.0795 ([M+H]
+), found
m/z 521.0791.; elemental analysis: calc'd. for (C
21H
20N
4O
8S
2): C, 48.46; H, 3.87; N, 10.76. found: C, 48.43; H, 3.94; N, 10.77.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The 1,2-diamine substructure is a ubiquitous structural motif that is found in numerous natural and biologically active compounds.
3,4 Thus, the development of method, capable of synthesizing 1,2-diamines with control of the stereochemistry, is desirable in term of preparing valuable compounds efficiently.
5 Of the various starting materials that are available for the formation of 1,2-diamines, alkenes represent suitable substrates
6 because they are easily accessible. In this context, stereoselective diamination of alkenes would be ideal approach to synthesis 1,2-diamines.
In 2021, our group reported the iodine-catalyzed stereospecific diamination of unactivated alkenes using nosylamide as a nitrogen source in conjunction with
NaOCl·5H2O.
7 The diamination proceeded with complete stereospecificity, yielding
anti-addition products from both
E and
Z alkenes (Table 1, entry 1-4). Cyclic alkenes were also diaminated without any detectable formation of a
syn-adduct (Table 1, entry 5 and 6).
Table 1. Representative Examples of Iodine-Catalyzed Diamination; Reaction Conditions: 40-80 ℃, 12-36 h. All yields are isolated yields
The two nosyl groups of the diaminated product could be readily detached, delivering the free 1,2-diamine (Scheme 1). This method, which produce only NaCl and H2O as by-products and operate without the need of any metals, is green-sustainable and suitable for diverse applications, particularly in the field of medicinal chemistry.
Scheme 1. Removal of Ns units to afford free 1,2-diamine
Appendix
Chemical Abstracts Nomenclature (Registry Number)
nosylamide: 2-nitrobenzendulfonamide; (5455-59-4)
Iodine: Iodine; (7553-56-2)
NaOCl·5H2O: Sodium hypochlorite pentahydrate; (10022-70-5)
trans-β-methylstyrene: trans-β-methylstyrene; (873-66-5)

|
Satoshi Minakata was born in Wakayama, Japan in 1964. He received his Ph.D. in 1993 from Osaka University (Japan) under the direction of Professor Yoshiki Ohshiro. After spending two years (1993-1995) at Central Research Laboratories of DIC Corporation (Japan), he was appointed as an assistant professor of Department of Applied Chemistry in Professor Komatsu group at Osaka University, and promoted to lecturer in 2000. From 1997 to 1998, he worked with Prof. Erick. M. Carreira at the California Institute of Technology (USA) as a visiting associate. In 2002, he was promoted to associate professor and since 2010, he has been a full professor at Osaka University. His research interest centers the development of new methodologies for synthesis of valuable organic molecules from simple molecules. |

|
Takeshi Sugiyama was born in Osaka, Japan in 1999. He received his B.S. (2022) and M.S. (2024) from Osaka University (Japan) under the supervision of Professor Satoshi Minakata. His research focused on a diastereodivergent diamination of a wide range of alkenes with haloamine reagents. He is currently engaged in drug discovery research at Shionogi & Co.,Ltd. |

|
Chiyuki Naito was born in Osaka, Japan in 2001. She received her B.S. in 2024 from Osaka University (Japan) under the supervision of Professor Satoshi Minakata. She is currently a master course student and perform her studies, working on the development of difunctionalization of vicinal C(sp3)-H bonds. |

|
Shun Kawano was born in Utsunomiya, Japan in 1999. He received his B.S. (2021) and M.S. (2023) from Tohoku University (Japan) under the supervision of Professor Hidetoshi Tokuyama. He is currently pursuing his Ph.D. at the same graduate school. His research interests are the area of the total synthesis of complex natural products. |

|
Juri Sakata was born in Shizuoka, Japan in 1986, and received his BSc (2009), MSc (2011) from Kogakuin University under the supervision of Professor Shinji Nagumo and Professor Masaaki Miyashita. He then moved to the laboratories of Professor Keisuke Suzuki at the Tokyo Institute of Technology and got his Ph. D. in 2015. In 2015, he joined the group of Professor Hidetoshi Tokuyama and was appointed Assistant Professor. His current research interest is total synthesis of complex natural product. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved