Org. Synth. 2007, 84, 359
DOI: 10.15227/orgsyn.084.0359
Practical Synthesis of a Chiral Ynamide: (R)-4-Phenyl-3-(2-triisopropylsilyl-ethynyl)oxazolidin-2-one
[2-Oxazolidinone, 4-phenyl-3-(2-triisopropylsilyl-ethynyl)-, (4R)-]
Submitted by I. K. Sagamanova, K. C. M. Kurtz, and R. P. Hsung
1.
Checked by Karen M. Marcantonio and David J. Mathre.
1. Procedure
A. 1-Bromo-2-triisopropylsilyl-ethyne (2). To a flame-dried single-necked 1-L round-bottomed flask equipped with a magnetic stir bar is added a solution of triisopropylsilylacetylene (22.0 g, 120.6 mmol) (Note 1) in anhydrous THF (500 mL) (Note 2). The solution is cooled to − 78 °C, and n-BuLi (50.7 mL, 126.7 mmol, 1.05 equiv) (Note 3) is added by syringe through the septum. The reaction is stirred for 30 min at − 78 °C, and Br2 (6.80 mL, 132.7 mmol, 1.10 equiv) (Note 4) is added slowly through the septum using a syringe. The reddish brown color from Br2 disappears as it is consumed upon addition. The solution remains reddish brown when the addition is complete. The mixture is stirred for 15 min at −78 °C and then quenched by addition of saturated aqueous Na2S2O3 (150 mL) after removal of the septum. The reaction mixture is transferred to a separatory funnel and the layers separated. The aqueous layer is further extracted with methyl tert-butyl ether (MTBE) (3 × 50 mL), and the combined organic extracts are washed with saturated aqueous NaCl (50 mL), dried over Na2SO4, filtered and concentrated on a rotary evaporator (20 – 30 mmHg, 50 °C) to yield the crude alkynyl bromide 2 (30.22–30.24 g, 96%) as a pale yellow oil (Note 5). Alkynyl bromide 2 is used without further purification (Note 6).
B. (R)-4-Phenyl-3-(2-triisopropylsilyl-ethynyl)oxazolidin-2-one (3). To a solution of 1-bromo-2-triisopropylsilylacetylene (2) (28.19 g, 107.9 mmol) (Note 7) in freshly distilled anhydrous toluene (100 mL) (Note 2) in a 250-mL, single-necked, round-bottomed flask fitted with a magnet stir bar are added R-phenyloxazolidinone (17.60 g, 107.9 mmol, 1.00 equiv) (Note 8), K2CO3 (29.81 g, 215.7 mmol, 2.00 equiv) (Note 9), CuSO4•5H2O (2.69 g, 10.8 mmol, 0.10 equiv) (Note 10), and 1,10-phenanthroline (3.89 g, 21.6 mmol, 0.20 equiv) (Note 11). The flask is fitted with a reflux condenser topped with a septum and N2 inlet, and heated in an oil bath at 75 °C (bath temperature) for 48 h. The reaction is monitored using TLC analysis (Note 12). Upon completion, the reaction mixture is cooled to room temperature and filtered through a 200-mL coarse-fritted vacuum filtration funnel containing a 5-cm layer of silica gel covered with a 1-cm layer of Celite. The mixture is washed through with 50% EtOAc/hexanes (400 mL), and the filtrate is concentrated on a rotary evaporator (20 – 30 mmHg, 70 °C). The crude residue is purified using silica gel column flash chromatography (Note 13) to give ynamide 3 (29.5–32.7 g, 81–88%) (Notes 14 and 15) as a yellow oil.
2. Notes
1.
Triisopropylacetylene (97%) was purchased from GFS Chemicals.
2.
Anhydrous solvents were obtained from an MBraun solvent purification system. Unstabilized
THF was purchased from JT Baker, and
toluene was purchased from Aldrich.
3.
N-Butyllithium (2.5 M solution in hexanes) was purchased from Aldrich Chemical Co.4.
Bromine (99+%) was purchased from Fisher Scientific.
5.
Characterization of
2: R
f = 0.72 [25% EtOAc in hexanes]; yellow oil;
1H NMR
pdf (300 MHz, CDCl
3) δ 1.06–1.10 (m, 21 H);
13C NMR
pdf (75 MHz, CDCl
3) δ 11.2, 18.4, 61.6, 83.4.
6.
This purification step was not necessary, because, in most cases, high purity was obtained judging from
1H NMR. However, product
2 was stable to silica gel column flash chromatography and could be eluted with hexanes. Other alkynyl bromides may require silica gel column flash chromatography for purification.
7.
The amidation reaction was performed successfully on a variety of scales [see Discussion section].
8.
R-Phenyloxazolidinone (98%) was purchased from Aldrich and used as received.
9.
Potassium carbonate (98%; ~ 325 mesh powder) was purchased from Aldrich.
10.
CuSO4•5H2O (99.3%) was purchased from J. T. Baker. The solid was ground into a powder with a mortar and pestle before use.
11.
1,10-Phenanthroline (99+%) was purchased from Aldrich. The solid was ground into a powder with a mortar and pestle before use.
12.
TLC analysis (25% EtOAc/hexanes): R
f(oxazolidinone) = 0.03, R
f (
2) = 0.72, R
f (
3) = 0.39.
13.
The crude product was dissolved in
200 mL of CH2Cl2 in a
round-bottomed-flask, and 75 g silica gel was added to the flask. The CH
2Cl
2 was removed on a rotary evaporator (20–30 mmHg, 35 °C) equipped with a bump trap. The dried contents of the flask were loaded onto the column (size
l ×
d = 30 cm × 5 cm) containing a slurry of silica gel and hexanes layered with sand. Gradient elution [EtOAc in hexanes]: 800 mL 0%, 800 mL 2%, 1000 mL 5%, 1500 mL 7%, 2000 mL 10%.
14.
GC analysis of ynamide
3 shows its purity to be =98.0%.
15.
Characterization of
3: R
f = 0.39 [25% EtOAc in hexanes]; clear oil; [α]
D23 − 133.7 (c 1.21, CH
2Cl
2); Chiralcel OD-H (250 × 4.6 mm), 5% IPA/heptane, 1.0 mL/min, 215 nm,
tR (
R) 10.77 min,
tR (
S) 13.66 min: >99.5 % ee R.
1H NMR
pdf (500 MHz, CDCl
3) δ: 0.90–0.93 (m, 21 H), 4.28 (dd, 1 H,
J = 7.6, 8.8 Hz), 4.73 (t, 1 H,
J = 8.8 Hz), 5.06 (dd, 1 H,
J = 7.6, 8.8 Hz), 7.34–7.45 (m, 5 H);
13C NMR
pdf (75 MHz, CDCl
3) δ: 11.0, 18.3, 62.2, 70.4, 71.9, 91.8, 127.1, 129.1, 129.4, 135.7, 155.2; IR (thin film) cm-1 2943 (m), 2185 (w), 1782 (s), 1394 (m), 883 (m); mass spectrum (APCI):
m/z (% relative intensity) 344 (13) (M + H)+, 334 (33), 318 (100);
m/z calcd for C
20H
29NO
2Si 344.2040, found 344.2047
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
It is note worthy that this cooper-catalyzed amidation of alkynyl bromide can be carried out with scales ranging from 48 to 107 mmol. The respective ynamide products were isolated with yields ranging from 86–89 %.
Table 1
The level of purity of the ynamides prepared using this procedure can be unambiguously established using GC/HPLC analysis for ynamide 3. The level of optical purity of the product is dependent upon the optical purity of the chiral auxiliaries acquired from commercial sources. The [α]D20 value for the commercial (R)-2-phenyloxazolidinone used in this preparation was −52.3 [c 2.0, CHCl3]. The [α]D20 values reported by Aldrich for (R)-2-phenyloxazolidinone and (S)-2-phenyloxazolidinone are −48.0 [c 2.0, CHCl3], which indicates that the ee or optical integrity of the Evans' auxiliary used for this work was very high. Severe erosion of the auxiliary's ee is unlikely under the amidation conditions described herein. The anticipated high ee of chiral ynamide (R)-3 was confirmed by chiral HPLC.
Ynamides
2–9 have become a highly attractive building block for developing synthetic methodologies.
10–11 As illustrated in Figure 1, there have been at least 30 reports in the last few years describing dozens of different strategies employing ynamides. Particularly attractive are those in which the nitrogen atom of ynamides becomes an integral part of various products that possess potential in alkaloid synthesis. These efforts demonstrate that there can be a distinct advantage in utilizing ynamides over simple alkynes. Therefore, the key issue, in part being addressed in this
Organic Syntheses procedure, is their preparation. We, as well as others, have exerted much effort toward this goal.
3–9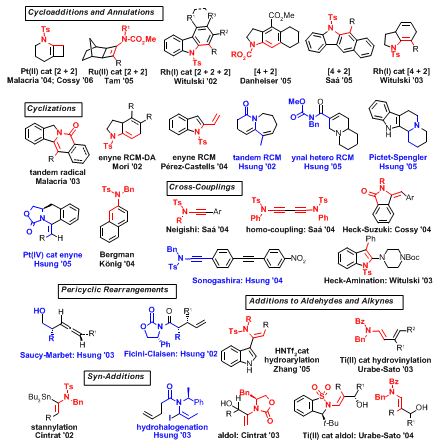
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
n-Butyllithium:
Butyllithium; (109-72-8)
Triisopropylsilylacetylene:
Silane, ethynyltris(1-methylethyl)-; (89343-06-6)
Copper sulfate pentahydrate; (7758-99-8)
1-Bromo-2-triisopropylsilyl-ethyne:
Silane, (bromoethynyl)tris(1-methylethyl)-; (111409-79-1)
R-Phenyloxazolidinone:
(4R)-4-Phenyl-2-Oxazolidinone; (90319-52-1)
1,10-Phenanthroline; (66-71-7)
 |
Richard P. Hsung was born in China in 1966. After growing up in New York City and Boston, he obtained his B.S. in Chemistry and Mathematics in 1988 from Calvin College. He attended The University of Chicago and received his Ph.D. degree in Organic Chemistry in 1994, under the supervision of Professors Jeff Winkler and Bill Wulff. He completed his training as an NIH post-doctoral fellow in Professor Gilbert Stork's laboratory at Columbia University. In 1997, he began his academic career at the University of Minnesota, moving to University of Wisconsin at Madison in 2006. He is a recipient of numerous awards including the Camille Dreyfus Teacher-Scholar Award and National Science Foundation Career Award. His current research focuses on the development of cycloaddition and annulation strategies to natural product syntheses.
|
 |
Irina K. Sagamanova was born in Noyabrsk, Russia, in 1985, and received a B.S. in Chemistry from The Higher Chemical College Russia Academy of Science in Moscow in 2006. She went to University of Minnesota and worked in Professor Richard P. Hsung's research group during the summer of 2005. Her research involves improving synthesis of chiral ynamides and exploring their reactivities.
|
 |
Kimberly C. M. Kurtz was born in Findlay, OH, in 1979, and received a B.S. in Chemistry from Ohio Northern University, Ada, OH in 2001. Her father, David Kurtz, was a long time Organic Chemistry faculty at ONU. She enrolled at University of Minnesota-Twin Cities and worked in Professor Richard P. Hsung's laboratory. Her research involved improving synthesis of chiral ynamides and exploring their reactivity in various pericyclic and ring-closing reactions. In 2006, she obtained her Ph.D. in Organic Chemistry, and is currently a senior scientist in the Corporate Research Laboratory at 3M in Saint Paul, MN.
|
 |
Karen Marcantonio was born and raised in Cranston, RI. She earned her BS in chemistry in 1997 at Connecticut College, doing research under Dr. Timo Ovaska and Dr. Bruce Branchini. After a summer internship at Pfizer, she began graduate school at UPenn, earning her masters from Marisa Kozlowski in 1999. She has been working in Merck Process Research since November 1999.
|
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved