Checked by Maurus Mathis, Jorge A. González, and Cristina Nevado
1. Procedure (Note 1)
A. 2,3,7,8-Tetrafluorothianthrene-S-oxide (2). In air, a three-necked 1000-mL round-bottomed flask (29/32) is charged with an egg-shaped Teflon coated magnetic stir bar (50 × 17 mm) and aluminium chloride (3.33 g, 25.0 mmol, 0.10 equiv) (Note 2). The central neck of the flask is fitted with a reflux condenser with the outlet passing through a round-bottomed flask (250 mL, 29/32) before entering a Drechsel bottle containing an aqueous sodium hydroxide solution (Note 3). Through a side neck, 1,2-difluorobenzene (0.250 L, 285 g, 2.50 mol, 10.0 equiv) (Note 4) is added to the 1000-mL round-bottomed flask. The flask is fitted with an adapter for the nitrogen inlet on the left neck (Note 5) and a 50 mL dropping funnel on the right neck. Disulfur dichloride (20.0 mL, 33.8 g, 0.250 mol, 1.00 equiv) (Note 6) is added to the addition funnel (Figure 1A), and then added drop-wise at room temperature (Note 7) over a period of 10 min to the stirred reaction mixture (Note 8). Upon addition of the disulfur dichloride, the solution becomes black (Figure 1B) and evolves HCl gas. After complete addition of the disulfur dichloride, the reaction mixture is heated at a gentle reflux (105 °C, bath temp)) using a silicon oil bath (Note 9) for 1 h. The oil bath is then replaced by an ice water bath, and MeOH (200 mL) is added to the reaction mixture via the addition funnel (Note 10). The dark color quickly dissipates from the reaction mixture, resulting in a light yellow suspension with a colorless solid (2,3,7,8-tetrafluorothianthrene) (Figure 1C).

Figure 1. A) Reaction set-up, B) the reaction mixture after disulfur dichloride addition, C) reaction mixture after the methanol quench, and D) crude 2,3,7,8-tetrafluorothianthrene (photos A, B, and C were provided by the submitters; photo D was provided by the checkers)
The slurry is stirred for 30 min in the ice water bath, and the solid material is subsequently filtered by vacuum filtration (Note 11). The filter cake is washed with chilled methanol (2 × 50 mL) (Notes 10 and 12), and the resulting off-white solid is transferred into a 500-mL one-necked, round-bottomed flask (29/32) and dried under vacuum (Note 13) for 18 h at room temperature. The resulting 23.82 g off-white solid (Figure 1D) contained 74.3% of 2,3,7,8-tetrafluorothianthrene by weight (Note 14), which is used without any further purification in the subsequent reaction (Notes 15 and 16).
In air, the 500-mL one-necked, round-bottomed flask (29/32) containing 23.82 g of crude 2,3,7,8-tetrafluorothianthrene (wt% = 74.3%; Note 14) is charged with an egg-shaped Teflon-coated magnetic stir bar (27 × 10 mm), DCM (175 mL, c = 0.35 M), Fe(NO3)3 ∙ 9H2O (42.2 g, 105 mmol, 1.7 equiv), NaBr (443 mg, 4.21 mmol, 0.07 equiv), and TFA (6.60 mL, 9.83 g, 86.2 mmol, 1.4 equiv) (Note 17). After addition, the flask is sealed with a rubber septum (Note 18). The resulting light brown suspension (Figure 2B) is stirred at room temperature for 20 h (Note 19). After this time, deionized water (150 mL) is added (Figure 2C).
Figure 2. The reaction mixture (A) before addition of TFA, (B) after addition of TFA, and (C) after addition of water (photos provided by submitters)
The suspension is filtered by vacuum filtration (Note 11), the filtrate is poured into a 500-mL separating funnel, and the layers are separated. The aqueous layer is extracted with DCM (2 × 150 mL). The filter cake is added to the combined organic layers, and the suspension is concentrated under reduced pressure (525 to 20 mmHg, 40 °C) (Note 20). MeCN (50 mL) (Note 21) is added to the residue, and the resulting suspension is filtered by vacuum filtration (Note 11). The solid is washed with MeCN (3 × 25 mL) (Note 12) (Figure 3A), which removes the majority of the red color. The solid is transferred into a 1000-mL one-necked, round-bottomed flask. Toluene (600 mL) (Note 22) is added, the flask is equipped with a reflux condenser, and the mixture is heated to reflux (110 °C) using a silicone oil bath. After 10 min at this temperature, heating and stirring is turned off and the mixture is left in the oil bath for 5 min. Subsequently, the mixture is decanted into a 1000-mL Erlenmeyer flask in order to remove the red-brown solid. Then the toluene solution is allowed to cool down to room temperature for 3 h. The obtained crystals (Figure 3B) are collected by vacuum filtration (Notes 11 and 23), washed with Et2O (2 × 50 mL) (Notes 12 and 24) (Figure 3C), and transferred into a 500-mL one-necked, round-bottomed flask. The filtrate is concentrated via rotary evaporator (50 mmHg, 40 °C), and acetone (600 mL) is added to the resultant solid (Note 25). The mixture is heated at 60 °C using a silicone oil bath for 5 min. After this time the mixture is allowed to stand for 5 min. Subsequently, the mixture is decanted into a 1000-mL one-necked, round-bottomed flask (29/32). The solution is concentrated via rotary evaporator (400 mmHg, 40 °C). The product is transferred into a 250-mL one-necked, round-bottomed flask (29/32) and recrystallized from toluene (80 mL, 110 °C) using a silicone oil bath. After cooling to room temperature for 1 h the colorless crystals are collected by vacuum filtration (Note 11), washed with Et2O (2 × 25 mL) (Note 12) (Figure 3D)and combined with the first batch of crystals in the 500-mL one-necked, round-bottomed flask (29/32). The combined batches are recrystallized again from toluene (240 mL, 110 °C) using a silicone oil bath. After cooling to room temperature for 2 h, the off-white crystals are collected by vacuum filtration (Note 11), washed with Et2O (2 × 50 mL) (Note 12) and ground into a fine powder using a porcelain mortar. The resulting off-white powder is transferred into a 100-mL one-necked, round-bottomed flask and is dried under vacuum for 18 h at room temperature to afford 11.33 g of 2,3,7,8-Tetrafluorothianthrene-S-oxide (2) with a purity of 98.3% (15 % yield over two steps) (Figure 3E) (Notes 26 and 27).
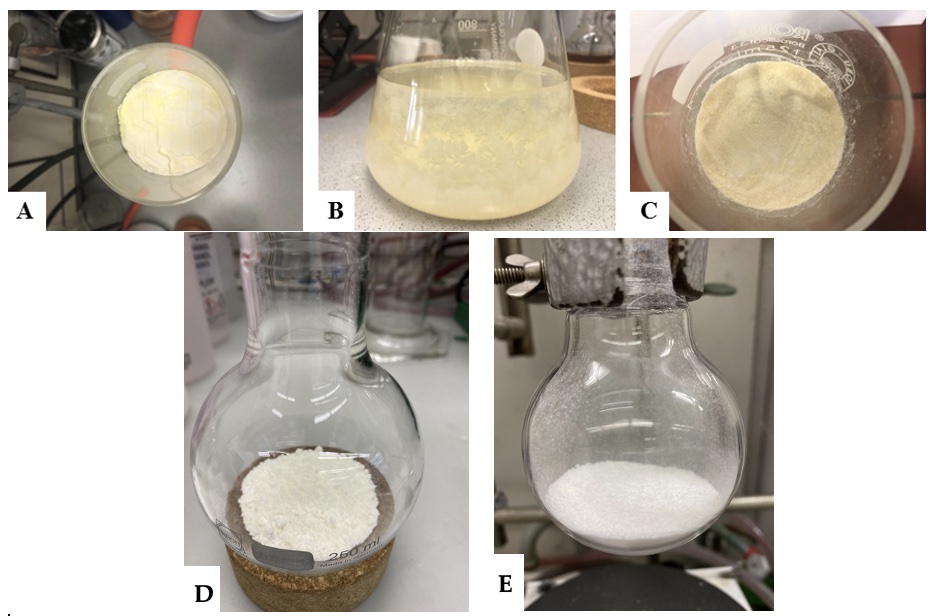
Figure 3. A) Appearance of the crude product, B) the crystals during recrystallization, C) the product after filtration, D) second batch crystals, and E) final recrystallized product (photos A, B, and C were provided by the submitters; photos D and E were provided by the checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
aluminium chloride,
sodium hydroxide,
1,2-difluorobenzene,
disulfur dichloride,
methanol,
2,3,7,8-tetrafluorothianthrene,
dichloromethane,
Iron(III) nitrate nonahydrate,
sodium bromide,
trifluoroacetic acid,
acetonitrile,
toluene,
diethyl ether,
acetone,
2,3,7,8-Tetrafluorothianthrene-S-oxide,
2-Phenylethyl acetate,
trifluoroacetic anhydride,
tetrafluoroboric acid diethyl ether complex,
isopropyl alcohol,
isohexane,
2-phenylethyl acetate derived tetrafluorothianthrenium salt,
4-biphenylboronic acid,
[1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II),
tripotass-ium phosphate,
1,4-dioxane,
ethyl acetate,
[4-(4-phenylphenyl)-phenyl] ethyl acetate; as well as the proper procedures for quenching gaseous
HCl which is evolving during a reaction.
2.
Aluminium chloride (anhydrous, granular, 99%) was obtained from Alfa Aesar and used as received.
3.
Sodium hydroxide (
NaOH) (99%) was purchased from VWR chemicals and used as received. The Drechsel bottle was filled with aqueous
NaOH solution (175 mL, 12.5 M). This
HCl quenching setup using
NaOH produces large amounts of heat and should be carried out with a glass container/flask rather than a plastic drying tube.
4.
1,2-Difluorobenzene (98%) was purchased from Fluorochem and used as received. The quantity of
1,2-difluorobenzene used in the reaction was consistent with its use as a solvent. When less
1,2-difluorobenzene was used, larger quantities of side-products resulted, which could not be removed by crystallization.
5.
Nitrogen was used to purge
HCl from the reaction flask and prevent backflow of the aqueous
sodium hydroxide solution. Alternatively, argon can be used.
6.
Disulfur dichloride (98%) was purchased from Sigma-Aldrich and used as received.
7. Room temperature throughout this manuscript refers to a temperature between 23 °C and 25 °C.
8. The stir plate was purchased from Heidolph Instruments GmbH & Co. KG. It has an input power of 230-240 V (50-60 Hz, 825 W), and the stirring range is from 100 rpm to 1400 rpm. Unless otherwise indicated, 500 rpm was used for stirring.
9. The silicone oil for the oil bath was purchased from abcr GmbH & Co. KG. The boiling point is over 205 °C. The oil in the oil bath should be level with the level of the reaction mixture in the reaction flask while heating. Unless otherwise indicated, the reported temperatures throughout this context refer to temperatures of oil in oil baths which were detected by the stirring plates' external temperature detectors. Approximately 15 min were necessary to increase temperature from 25 to 105 °C.
10.
Methanol (
MeOH, ≥ 99.8%) was purchased from Sigma-Aldrich and used as received. In order to precool the
methanol, the required amount was placed in a 500 mL Erlenmeyer flask and cooled in an ice-water bath to 2-5 °C for 10 min. The addition was performed in 50 mL portions via the addition funnel.
11. Büchner funnel VitraPOR® with Por. 4 and a capacity of 125 mL was used.
12. To rinse the impurities out of the flask, manual stirring with a stainless spatula was applied to thoroughly mix the rinse solvent with the solid. When the filtration was complete, the filter cake was pressed with the top of a glass stopper to remove as much liquid as possible.
13. The vacuum pump was supplied by Vacuubrand GmbH & Co. KG. Vacuum refers to pressure lower than 0.075 mmHg.
14. Quantitative
19F NMR
pdf (471 MHz, CDCl
3) was performed using
4,4'-difluorobenzophenone (≥ 99% purity, Sigma Aldrich) as an internal standard. The NMR sample was prepared by dissolving
4,4'-difluorobenzophenone (14.3 mg) and
2,3,7,8-tetrafluorothianthrene (10.3 mg) in CDCl
3 (0.5 mL) in a 5 mm NMR tube. The sample was subsequently analyzed by
19F NMR spectroscopy with the following parameters: no. of scans: 32, D1: 20 s, rotation frequency: 15 Hz, spectral width: 49.7924 ppm, transmitter frequency offset: -121.3 ppm). Integration of the fluorine signals showed a 1:0.81 ratio, corresponding to 74.3 wt% of
2,3,7,8-tetrafluorothianthrene.
15. The off-white solid has the following characteristics:
1H NMR
pdf (500 MHz, CDCl
3) δ: 7.32 (t,
J = 8.5 Hz, 4H).
19F NMR
pdf (471 MHz, 298 K, CDCl
3) δ: -136.86 (t,
J = 8.6 Hz).
16. Purification of
2,3,7,8-tetrafluorothianthrene: A column (1 cm diameter) with a glass frit was charged with copper powder (2.9 g, 4.56 mmol) (powder, 99.999%, trace metal basis, Sigma Aldrich) The column was purged with argon, then, with the stopcock closed, 7 mL of concentrated
HCl (37-38%, JT-Baker) was added to the copper powder, put under argon atmosphere, and gently agitated to liberate gas bubbles. After 5 min the stopcock was opened, and a positive pressure of argon was applied so that the concentrated
HCl was eluted at a rate of about two drops∙s
-1 (total elution time about 2 min). The copper bed was washed twice with 10 mL of Milli-Q water by applying a positive pressure of argon (2 drops/s, about 2 min elution time for each wash).
Nitrogen was allowed to flow through the column-bed for 5 min to remove as much water as possible. Then the copper was washed twice with 10 mL of anhydrous
acetone using a positive pressure of argon (99.8%, ExtraDry, AcroSeal
TM, purchased from Fisher Scientific; about 2 min elution time for each wash). Again, argon was allowed to flow through the bed for 5 min to remove as much
acetone as possible. Argon pressure was released, and the stopcock was closed. Then a solution of 320 mg of contaminated TFT in 10 mL of
DCM was added to the column and allowed to sit with the stop cock closed for 10 min (all under argon atmosphere). At this point, the sample was allowed to elute into a 50 mL round-bottomed flask (approx. 10 min). The resulting column bed of black solid was washed with 10 mL of
DCM using a positive pressure of argon and collected in the same 50 mL round-bottomed flask as the first
DCM elution (about 2 min elution time). The combined
DCM elutions were concentrated via rotary evaporator, and the colorless solid was dried under high vacuum at room temperature for 5 h yielding 225 mg of TFT with a purity of 98% (determined by quantitative
19F-NMR
pdf). Purified
2,3,7,8-tetrafluorothianthrene (colorless solid) has the following characteristics:
1H NMR
pdf (500 MHz, CDCl
3) δ: 7.32 (t,
J = 8.5 Hz, 4H).
13C NMR
pdf (126 MHz, CDCl
3) δ: 117.8 (dd,
J = 13.8, 6.7 Hz), 131.5 (t,
J = 5.0 Hz), 150.2 (dd,
J = 254.3, 15.2 Hz).
19F NMR
pdf (471 MHz, 298 K, CDCl
3) δ: -136.86 (t,
J = 8.6 Hz). IR (neat): 3096, 3073, 3039, 2194, 2159, 2130, 2037, 2007, 1995, 1972, 1730, 1589, 1562, 1466, 1373, 1277, 1221, 1193, 1089, 969, 874, 852, 791, 679, 617, 587, 463, 440, 424 cm
-1. EI-HRMS (
m/z) calcd. for C
12H
4S
2F
4 [M]
+ 287.96850 found 287.96848. mp = 199.7 °C. The compound is bench stable.
17.
Dichloromethane (
DCM) (≥99%) was purchased from Sigma-Aldrich. Fe(NO
3)
3 ∙ 9H
2O (98+%, metal basis) was purchased from Alfa Aesar.
Sodium bromide (99.5%) was purchased from Riedel-de Haen.
TFA (99%) was purchased from abcr GmbH & Co. They were all used as received.
18. The rubber septum was equipped with a short needle to prevent over-pressure. The reaction was performed under air.
19. The progress of the reaction was followed by TLC analysis on silica gel (POLYGRAM® SIL G/UV with 0.20mm silica gel 60 with fluorescent indicator, purchased from Macherey-Nagel) with
EtOAc-
isohexane 1:10 (v/v) as eluent and visualized with
KMnO4-stain. The tetrafluorothianthrene starting material has R
f= 0.65 and the
tetrafluorothianthrene-S-oxide product has R
f= 0.23.
Figure 7. TLC of starting material (SM) versus reaction spot of the end of reaction (P), a central co-spot (Co) and a spot for the product (R). (photo provided by submitters)
20. A BUCHI Vacuum Controller V-850 in combination with evaporator R-210 was used for rotary evaporation. Water was used in the heating bath. Unless otherwise indicated, the pressure and temperature were read from the controller.
21.
Acetonitrile (
MeCN) (≥ 99.9%) was purchased from Sigma-Aldrich and used as received.
22.
Toluene (tech. grade) was purchased from OQEMA GmbH and used as received.
23. The filtrate was collected in a 1000-mL one-necked, round-bottomed flask.
24.
Diethyl ether (
Et2O) (for analysis EMSURE® ACS, ISO, Reag. Ph Eur) was purchased from Sigma-Aldrich and dried over sodium-potassium alloy before use.
25.
Acetone (tech. grade) was purchased from OQEMA GmbH and used as received.
26. The off-white solid has the following characteristics:
1H NMR
pdf (400 MHz, CDCl
3) δ: 7.49 (dd,
J = 9.0, 6.5 Hz, 2H), 7.73 (dd,
J = 8.8, 7.3 Hz, 2H).
13C NMR
pdf (101 MHz, CDCl
3) δ: 114.4 (dd,
J = 21.3, 1.6 Hz), 118.9 (d,
J = 20.4 Hz), 124.3 (dd,
J = 7.2, 4.1 Hz), 138.4 (t,
J = 3.7 Hz), 150.1 (dd,
J = 24.4, 13.3 Hz), 152.7 (dd,
J = 24.7, 13.3 Hz).
19F NMR
pdf (471 MHz, CDCl
3) δ: -132.82 (ddd,
J = 20.2, 9.0, 7.3 Hz), -133.66 (ddd,
J = 20.2, 8.8, 6.5 Hz). IR (neat): 3092, 3036, 1596, 1573, 1461, 1382, 1270, 1213, 1191, 1100, 1064, 955 cm
-1. EI-HRMS (
m/z) calcd. for C
12H
4O
1S
2F
4 [M]
+ 303.9634, found 303.9634. mp = 254.8 °C. The compound is bench stable. Quantitative
19F NMR
pdf (471 MHz, CDCl
3) was performed using
4,4'-difluorobenzophenone (≥ 99% purity, Sigma Aldrich) as an internal standard. The NMR sample was prepared by dissolving
4,4'-difluorobenzophenone (7.4 mg) and
2,3,7,8-Tetrafluorothianthrene-S-oxide (7.4 mg) in CDCl
3 (0.5 mL) in a 5 mm NMR tube. The sample was subsequently analyzed by
19F NMR spectroscopy with the following parameters: no. of scans: 32, D1: 20 s, rotation frequency: 15 Hz, spectral width: 49.7923 ppm, transmitter frequency offset: -119.0 ppm). Integration of the fluorine signals showed a 1:0.705 ratio, corresponding to 97.6 wt% of
2,3,7,8-tetrafluorothianthrene-S-oxide.
27. The reaction (Step A) was also checked on half-scale and provided 5.94 g (15%) of the same product with purity of 97%.
28.
2-Phenylethyl acetate (98%) and
TFAA (99+%) were purchased from Alfa Aesar and used as received.
29.
Tetrafluoroboric acid diethyl ether complex (51.0 - 57.0 wt% HBF
4) was purchased from Sigma Aldrich and used as received.
30. The progress of the reaction was followed by TLC analysis on silica gel (POLYGRAM® SIL G/UV with 0.20mm silica gel 60 with fluorescent indicator, purchased from Macherey-Nagel) with
DCM as eluent and visualized by 254 nm UV-light. The
tetrafluorothianthrene-S-oxide starting material has R
f = 0.55 and
2-phenylethyl acetate has R
f = 0.65.
Figure 8. TLC of starting material tetrafluorothianthrene-S-oxide (SM TFTO, left) and starting material 2-phenylethyl acetate (SM, right) versus reaction spot of the end of reaction (P). A central co-spot (Co) for both is also shown (photo provided by submitters)
31.
Isopropyl alcohol (
iPrOH) (tech. grade) was purchased from OQEMA GmbH and used as received.
32.
Isohexane (tech. grade) was purchased from OQEMA GmbH and distilled before use.
33. Flash column chromatography: A column (length: 26 cm, diameter: 8 cm, with a 1000 mL reservoir) was charged with 300 g SiO
2 (Geduran® Si 60 with pore size 40-63 µm from Merck KGaA) and 750 mL
DCM. In order to dry load the crude
2-phenethyl acetate-derived tetrafluoro-thianthrenium salt, it was dissolved in 300 mL
DCM at 40 °C using a water bath and 60 g of SiO
2 was added. The solvent was removed under reduced pressure (525 mmHg to 15 mmHg, 40 °C), and the dry residue was transferred on the column inside a fume hood. Sand (200 g) was added on top of the silica column bed. The product was eluted with 600 mL
DCM. At that point, fraction collection was initiated, and elution was continued with 500 mL
DCM/
i-PrOH 100:1 (v/v), 8 L
DCM/
i-PrOH 50:1 (v/v) and 2 L
DCM/
i-PrOH 10:1. Fractions were collected in 50 mL test tubes. TLC analysis of the product was done with
DCM/
i-PrOH 10:1 (v/v) as eluent and visualized with 254 nm UV light. Product could be found in fractions 41 to 219.
34. The colorless solid has the following characteristics:
1H NMR
pdf (400 MHz, CD
3CN) δ: 1.91 (s, 3H), 2.96 (t,
J = 6.5 Hz, 2H), 4.22 (t,
J = 6.5 Hz, 2H), 7.11 - 7.17 (m, 2H), 7.38 - 7.42 (m, 2H), 7.95 (dd,
J = 9.9, 7.0 Hz, 2H), 8.38 (dd,
J = 9.1, 7.2 Hz, 2H).
13C NMR
pdf (101 MHz, CD
3CN) δ: 21.0, 35.1, 64.5, 115.4 (dd,
J = 7.1, 3.6 Hz), 121.2 (d,
J = 21.9 Hz), 121.2, 125.5 (dd,
J = 22.0, 2.6 Hz), 129.3, 132.2, 135.2 (dd,
J = 8.0, 3.9 Hz), 146.3, 151.6 (dd,
J = 255.4, 13.3 Hz), 154.8 (dd,
J = 261.9, 13.2 Hz), 171.4.
19F NMR (471 MHz, CD
3CN) δ: -125.42 (ddd,
J = 20.2, 10.0, 7.1 Hz), -133.80 (ddd,
J = 20.1, 9.1, 7.0 Hz), -151.70 (br,
10B), -151.75 (br,
11B). IR (neat): 3093, 3048, 1726, 1574, 1481, 1380,1284, 1267, 1236, 1199, 1055, 1026, 1008, 965, 904 cm
-1. ESI-HRMS: (
m/z) calcd. for C
22H
15O
2S
2F
4 [M]
+ 451.0444, found 451.0444. mp = 207.0 °C. The compound is bench-stable. Quantitative
19F NMR
pdf (471 MHz, CD
3CN) was performed using
4,4'-difluorobenzophenone (≥ 99% purity, Sigma Aldrich) as an internal standard. The NMR sample was prepared by dissolving
4,4'-difluorobenzophenone (10.8 mg) and
2-phenethyl acetate derived tetrafluorothianthrenium salt (10.8 mg) in CD
3CN (0.5 mL) in a 5 mm NMR tube. The sample was subsequently analyzed by
19F NMR
pdf spectroscopy with the following parameters: no. of scans: 32, D1: 20 s, rotation frequency: 15 Hz, spectral width: 49.7924 ppm, transmitter frequency offset: -121.3 ppm). Integration of the fluorine signals showed a 1:0.405 ratio, corresponding to 99.9 wt% of
2-phenethyl acetate derived tetrafluorothianthrenium salt.
35. The reaction (Step B) was also checked on half-scale and provided 6.75 g (64%) of the same product.
36. Alternatively, the reaction can be carried out under argon atmosphere.
37.
4-Biphenylboronic acid (95%) was purchased from Oxchem,
Pd(dppf)Cl2 (98%) was purchased from fluorochem,
K3PO4 (97%) was purchased from Alfa Aesar, and
1,4-dioxane (≥ 99.5%) was purchased from Fisher Scientific. They were used as received.
38. The progress of the reaction was followed by TLC analysis on silica gel (POLYGRAM® SIL G/UV with 0.20mm silica gel 60 with fluorescent indicator, purchased from Macherey-Nagel) with
DCM-
iPrOH 10:1 (v/v) as eluent and visualized by 254 nm UV-light. The
2-phenethyl acetate derived tetrafluorothianthrenium salt starting material has R
f = 0.47, and the Suzuki coupling product
[4-(4-phenylphenyl)phenyl] ethyl acetate has R
f = 0.80.
Figure 9. TLC of starting material (SM) versus reaction spot of the end of reaction (P). A central co-spot (Co) and a spot for the clean product (R) is also shown (photo provided by submitters)
39.
Ethyl acetate (98-100%) was purchased from OQEMA GmbH and distilled before use.
40. Flash column chromatography: A column (length: 26 cm, diameter: 8 cm, with a 1000 mL reservoir) was charged with 300 g SiO
2 (Geduran® Si 60 with pore size 40-63 µm from Merck KGaA) and 800 mL Hex/
DCM 8:1 (v/v). In order to dry load the crude
[4-(4-Phenylphenyl)phenyl] ethyl acetate was dissolved in 250 mL
DCM at 40 °C using a water bath and 50 g of SiO
2 was added. The solvent was removed under reduced pressure (525 mmHg to 15 mmHg, 40 °C), and the dry residue was transferred on the column. Sand (200 g) was added on top of the column bed. The crude product is eluted with 1 L of a hexanes/
DCM mixture (4:1 v/v) and 3.2 L (hexanes /
DCM = 3:1 v/v). At that point, fraction collection is begun, and elution is continued with 4 L
DCM/hexanes 1:1 (v/v) and 4 L
DCM. Fractions were collected in 50 mL test tubes. TLC analysis of the product was done with
DCM/
i-PrOH 10:1 (v/v) as eluent and visualized with 254 nm UV light. Product could be found in fractions 44 to 120.
41. Büchner funnel VitraPOR® with Por. 4 and a capacity of 75 mL was used.
42. The off-white solid has the following characteristics:
1H NMR
pdf (400 MHz, CDCl
3) δ: 2.08 (s, 3H), 3.00 (t,
J = 7.1 Hz, 2H), 4.35 (t,
J = 7.0 Hz, 2H), 7.31 - 7.35 (m, 2H), 7.35 - 7.41 (m, 1H), 7.47 (dd,
J = 8.4, 6.9 Hz, 2H), 7.58 - 7.62 (m, 2H), 7.63 - 7.67 (m, 2H), 7.68 (s, 4H).
13C NMR
pdf (101 MHz, CDCl
3) δ: 21.1, 34.9, 65.0, 127.2, 127.2, 127.5, 127.6, 127.6, 128.9, 129.5, 137.1, 139.1, 139.9, 140.2, 140.8, 171.2. IR (neat): 3032, 2955, 2894, 1732, 1484, 1400, 1383, 1366, 1233, 1030, 1001, 980, 829, 819, 691 cm
-1. ESI-HRMS (
m/z) calcd. for C
22H
20O
2Na
1 [M + Na]
+ 339.1356, found 339.1357. mp = 168.1 °C. The compound is bench stable. Quantitative
1H NMR
pdf (500 MHz, CDCl
3) was performed using benzyl benzoate (certified reference material, TraceCERT®, Sigma Aldrich) as an internal standard. The NMR sample was prepared by dissolving benzyl benzoate (10.1 mg) and
[4-(4-phenylphenyl)phenyl] ethyl acetate (9.80 mg) in CDCl
3 (0.5 mL) in a 5 mm NMR tube. Integration of the proton signals showed a 1:0.65 ratio, corresponding to 99.9 wt% of
[4-(4-phenylphenyl)phenyl] ethyl acetate.
43. The reaction (Step C) was also checked on half-scale and provided 2.69 g (68%) of the same product.
3. Discussion
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved